Abstract
Background
For several types of cancer, including gastric cancer (GC), tumor cells at the invasive front are considered to have a more aggressive behavior compared with those in the more central region. The aim of the present study was to analyze the expression of MMP-7, laminin γ2 and EGFR in a large number of GCs and to investigate how these expression patterns correlate with clinicopathologic parameters, infiltrative patterns, histology or mucin phenotype.
Methods
We immunohistochemically examined the expression of MMP-7, laminin γ2 and EGFR using a tissue microarray analysis of 790 GCs, and evaluated their clinicopathological significance.
Results
MMP-7, cytoplasmic laminin γ2, extracellular laminin γ2 and EGFR expression were observed in 25, 25, 8 and 21 % of the 790 GC cases, respectively. Expression of MMP-7, cytoplasmic laminin γ2 and EGFR was associated with advanced T grade, N grade and tumor stage. Extracellular laminin γ2 expression was not associated with any clinicopathologic parameters, infiltrative patterns, histology or mucin phenotype. Furthermore, we investigated the correlations of MMP-7, laminin γ2 and EGFR expression. MMP-7 expression was significantly more frequent in positive expression of cytoplasmic laminin γ2 than negative cases, and EGFR expression was significantly more frequent in positive expression of cytoplasmic laminin γ2 and MMP-7.
Conclusions
Molecular expression of MMP-7, laminin γ2 or EGFR, and their combinations, may be associated with GC tumor aggressiveness. Assessment of expression of these molecules at the invasive front of primary tumors is clinically significant in predicting the malignant behavior of GC.
Similar content being viewed by others
Introduction
Gastric cancer (GC) is one of the most common malignancies worldwide and develops as a result of multiple genetic and epigenetic alterations [1]. Advances in diagnostic tools and treatments have led to excellent long-term survival for early-detected GC [2]. However, despite improvements in diagnostic and therapeutic strategies, the prognosis of advanced GC with extensive invasion and metastasis remains poor. Several discrete steps can be discerned in the biological cascades of metastasis [3], and several molecules have been suggested to be involved in mediating GC aggressiveness [4]. The histological features of GC may differ widely from area to area within the same tumor due to tumor heterogeneity. The most useful clinicopathologic features and molecular signatures can be deduced from the invasive front of the tumor, where the most transformed and presumably most aggressive cells reside. In addition to classification by histology (the Lauren classification, the Japanese Classification of Gastric Carcinoma, and so on), GCs may also be classified into four phenotypes by their mucin expression profile: G type (gastric phenotype), I type (intestinal phenotype), GI type (gastric and intestinal mixed phenotype) and N type (neither gastric nor intestinal phenotype). Although G-type tumors are associated with poor patient outcome and greater malignant potential in the incipient phase of invasion and metastasis compared with other types [5], there is little understanding of whether or not mucin phenotypic classification could be used for evaluating tumor aggressiveness at the invasive front of GCs.
In carcinomas, the basement membrane, a specialized form of extracellular matrix (ECM) that separates the tumor from the stroma and acts as a mechanical barrier against cancer cell invasion, must first be degraded to allow these cells to migrate [6]. Degradation of ECM components is mostly controlled by proteolytic enzymes called matrix metalloproteinases (MMPs). MMPs have been shown to be overexpressed in several kinds of carcinomas, and to be associated with tumor invasion, metastasis or progression [7]. MMP-7, also known as matrilysin, is a member of the MMP gene family and has proteolytic activity against a wide spectrum of substrates such as collagens, proteoglycans, elastin, laminin, fibronectin, and casein [8–10]. MMP-7 is often overexpressed at the invasive front in various types of human cancer and is associated with cancer progression [11, 12]. Previous reports have suggested that MMP-7 expression also correlates with tumor invasion and metastasis in advanced GC [13]. Laminins are a family of high–molecular weight ECM proteins, also involved in cellular adhesion, growth and differentiation [14]. Laminins consist of α, β, and γ chains, and there are at least 12 isoforms. Laminin-5, which consists of α3, β3, and γ2 chains, is localized in epithelial basement membranes, functions as a ligand for integrins, and plays an important role in cell migration and adhesion [15, 16]. Some studies have reported that laminin γ2 is expressed at the invasive front in tumor cells, while others demonstrated that loss of laminin γ2 in the epithelium-stroma interface is an immunohistochemical marker of malignancy in epithelial lesions [17–21]. Laminin γ2 expression patterns are divided into two distinct types, cytoplasmic staining and extracellular staining. Okada et al. [22] reported that stromal laminin γ2 expression is associated with poor prognosis and destructive growth of gallbladder adenocarcinoma. In GC, it has been also reported that cytoplasmic laminin γ2 staining is associated with advanced lymph node metastasis and tumor stage [23]. It has been reported that MMP-7 expression is correlated with laminin γ2 expression in colorectal and biliary tract cancer [21, 24]. However, little is known about the association between MMP-7 and laminin γ2 at the invasive front of GC. In addition, it has also been reported that the laminin γ2 chain is cleaved by membrane-type 1 MMP (MT1-MMP, MMP-14) and MMP-2 [25] and that the cleaved γ2 chains bind epidermal growth factor receptors (EGFR) on cancer cell surfaces and transmit intracellular signals that promote cell growth and mobility [26]. Furthermore, it has been reported that laminin γ2 expression is correlated with EGFR in oral [27–29] and esophageal [19] squamous cell carcinoma and lung adenocarcinoma [30].
Although MMP-7, laminin γ2 and EGFR are representative molecules recognized as independent prognostic markers, there is little understanding of the correlations with some of the possible combinations, and the relationship between the combination of markers and clinicopathologic factors. The aims of the present study were to analyze the expression of MMP-7, laminin γ2, EGFR or their combinations at the invasive front in a large number of GCs and to investigate how these expression patterns correlate with clinicopathologic parameters, infiltrative patterns, histology or mucin phenotype. Because the functional and biological properties of GCs may reflect the tumor’s ability to produce these molecules, it would be of interest to determine which factors are best correlated with tumor aggressiveness.
Materials and methods
Samples of GCs at the invasive front and tissue microarray (TMA) construction
We randomly selected a total of 1019 GCs from the surgical pathology files of the Hiroshima University Hospital and its affiliated hospitals. Among those, 229 cases (22 %) were intramucosal GCs and were excluded from the present study, leaving 790 GCs (78 %) diagnosed with pathologically proven invasive GCs (507 men and 283 women; age range, 31–93 years). Surgically resected specimens were routinely fixed in 10 % buffered formalin and examined macroscopically. All sections contained tumor tissue and surrounding non-neoplastic tissue and were embedded in paraffin. Additional consecutive 5-μm sections were cut from a selected tissue block and stained with hematoxylin and eosin (HE). Tumor staging was performed according to the Union Internationale Contre le Cancer (UICC) system [31]. There were 248 T1 and 542 T2–T4 in these 790 cases. Nodal metastasis and distant metastasis were present in 428 patients and 8 patients (54 and 1 %, respectively). Tumor staging revealed 352 stage I and 438 stage II–IV cases. The 790 GC cases were histologically classified as 436 intestinal type and 354 diffuse type, according to the Lauren classification system [32]. Using the Japanese Classification of Gastric Carcinoma, tumor infiltration patterns (INFs) were classified into three subgroups according to the pattern of tumor infiltration into the surrounding tissue: INFa, INFb and INFc. The INFa group exhibits expanding growth and a distinct border with the surrounding tissue, INFc describes infiltrating growth and an indistinct border with the surrounding tissue, while INFb falls between the two (Fig. 1a–f). In accordance with the Ethical Guidelines for Human Genome/Gene Research enacted by the Japanese Government, tissue specimens were collected and used after approval by the Ethical Review Committee of the Hiroshima University School of Medicine and by the ethical review committees of collaborating organizations. The two most representative portions to be sampled for the TMAs were carefully selected from different intratumoral areas in each case and marked on the HE-stained slide. One invasive front area and one superficial area as its control were selected.
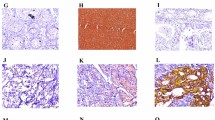
Infiltration pattern (a–f) at the invasive front of gastric cancer (GC) and immunostaining of MMP-7, laminin γ2 and EGFR (g–l). Tumor infiltration patterns (INFs) were classified into three subgroups according to the pattern of tumor infiltration into the surrounding tissue: INFa (a, d), INFb (b, e) and INFc (c, f). Immunohistochemically, MMP-7 was often coexpressed with cytoplasmic laminin γ2 (g, j), but rarely coexpressed with extracellular laminin γ2 (h, k). EGFR expression was also colocalized with cytoplasmic laminin γ2 (i, l)
The invasive front of GCs varies in complexity from smooth to highly complex when the front splits up into small cell clusters or even single cancer cells. In this study, we defined the invasive front of GCs as tumor cells or clusters at the perpendicularly deepest site of tumor invasion. A 2-mm-diameter tissue core of each donor block was punched out and transferred to a recipient block with a maximum of 48 cores using a tissue microarrayer (AZUMAYA KIN-1, Tokyo, Japan). Five-μm-thick sections were cut from the recipient block and transferred to slide glasses. HE staining was performed on TMA for confirmation of the tumor tissue.
Immunohistochemistry
For immunostaining of all markers except EGFR, a Dako LSAB Kit was used according to the manufacturer’s recommendations. The antibodies and their conditions used in the current study are shown in Table 1. After peroxidase activity was blocked with 3 % H2O2-methanol for 10 min, the sections were incubated with normal goat serum (Dako Corporation, Carpinteria, CA) for 20 min to block nonspecific antibody binding sites. The sections were incubated with the primary antibodies for 1 h at room temperature, followed by incubations with biotinylated anti-mouse immunoglobulin G and peroxidase-labeled streptavidin for 10 min each. For immunostaining of EGFR, a Dako EGFR pharmDx™ assay detection system (Dako Corporation, Carpinteria, CA) was used. Staining was completed with a 10-min incubation with the substrate-chromogen solution. The sections were counterstained with 0.1 % hematoxylin. Appropriate positive and negative control samples were also stained.
Evaluation of positive cases and cutoff-point thresholds
For the TMAs, staining was considered positive if any tumor cells were stained appropriately. The percentage of reactive cells necessary for a positive result reflects the viewpoint and opinion of the authors. Immunostaining results were evaluated independently by two investigators (KS and MM), and when the evaluations differed, a decision was made by consensus while investigators reviewed the specimen with a multihead microscope. Neoplastic tissue was evaluated semiquantitatively at magnifications of ×100 and ×400. The cytoplasmic staining of MMP-7, MUC5AC, MUC6 and MUC2, cytoplasmic and extracellular staining of laminin γ2, and the membranous staining of EGFR and CD10 were classified according to the percentage of stained cells within carcinomatous areas. The extracellular staining of laminin γ2 was characterized by the laminin γ2-positive staining in the stroma adjacent to the cancer cell nests. The expression of each molecule was classified as 0 % (score 0), 1–9 % (score 1), 10–49 % (score 2) or >50 % (score 3) of staining. When each specimen had more than 10 % (score 2 and 3) of cancer cells or stromal positively stained, the immunostaining was considered positive according to median cut off values rounded off to the nearest 5 %.
Mucin phenotypes of GCs
790 GCs were evaluated according to the criteria [33] for classification of G type and I type. GCs in which more than 10 % of the cells displayed the gastric (MUC5AC and/or MUC6) or intestinal epithelial cell phenotype (MUC2 and/or CD10) were considered G type or I type, respectively. Those sections that showed both G and I type were classified as GI type, and those that lacked both G and I type were classified as N type.
Statistical methods
Associations between clinicopathologic variables and immunostaining for MMP-7, laminin γ2 or EGFR were analyzed by the chi-square test. A p-value less than 0.05 was considered statistically significant. Hierarchical clustering analysis was performed using the WARD clustering algorithms. Statistical analyses were performed using JMP software (version 10.0.2; SAS Institute, Carey, NC).
Results
Staining patterns of MMP-7, laminin γ2 and EGFR at the invasive front and the control regions of GCs and their correlation with clinicopathologic parameters
We performed immunostaining of MMP-7, laminin γ2 and EGFR at the invasive front and the control regions of GCs. The median percentage of positive cancer cells was 9 (range 0–85) for MMP-7, 8 (range 0–70) for laminin γ2, and 8 (range 0–65) for EGFR.
At the invasive front of GCs, MMP-7 expression was detected in 195 (25 %) of the 790 cases (score 0: 122 cases, score 1: 473 cases, score 2: 177 cases, score 3: 18 cases) and was seen exclusively in the cytoplasm (Fig. 1g, h). Two laminin γ2 staining patterns (cytoplasmic staining and extracellular staining) have been reported in GCs [18, 23]. Laminin γ2 cytoplasmic expression was detected in 195 (25 %) (score 0: 156 cases, score 1: 439 cases, score 2: 182 cases, score 3: 13 cases) (Fig. 1j, l), and laminin γ2 extracellular expression was detected in 60 (8 %) (score 0: 302 cases, score 1: 428 cases, score 2: 54 cases, score 3: 6 cases) (Fig. 1k). EGFR membranous expression was detected in 162 (21 %) (score 0: 214 cases, score 1: 414 cases, score 2: 152 cases, score 3: 10 cases) of the 790 cases.
Next, we investigated the relationship between their expressions and clinicopathologic parameters including age, sex, T grade, N grade, M grade and tumor stage (Table 2). Expression of MMP-7 was associated with advanced T grade (p = 0.0207), N grade (p < 0.0001) and tumor stage (p < 0.0001). Expression of cytoplasmic laminin γ2 was associated with advanced T grade (p = 0.0003), N grade (p < 0.0001) and tumor stage (p < 0.0001). Expression of EGFR was associated with advanced T grade (p < 0.0001), N grade (p < 0.0001) and tumor stage (p < 0.0001). However, extracellular laminin γ2 expression was not associated with any clinicopathologic parameters.
In contrast, we performed immunostaining of MMP-7, laminin γ2 and EGFR at the superficial areas of GCs. MMP-7 expression was detected in 116 (15 %) of the 790 cases. Laminin γ2 cytoplasmic expression was detected in 145 cases (18 %), and laminin γ2 extracellular expression was detected in 41 cases (5 %). EGFR expression was detected in 69 (9 %) of the 790 GC cases. Expression of cytoplasmic laminin γ2 was associated with advanced T grade (p = 0.0074), N grade (p = 0.0096) and tumor stage (p = 0.0336), whereas MMP-7, extracellular laminin γ2 and EGFR expression were not associated with any clinicopathologic parameters (Table 3).
Correlation of MMP-7, laminin γ2 and EGFR expression with infiltrative patterns, histology and mucin phenotypes at the invasive front of GCs
We analyzed the relationships between expression of these molecules and infiltrative patterns, histology and mucin phenotypes at the invasive front of GC. Infiltrative patterns of 790 GCs included 122 INFa, 415 INFb and 253 INFc, and tumor histology was classified into 436 intestinal type and 354 diffuse type. The distribution of each mucin phenotype included 235 G type, 99 GI type, 167 I type and 289 N type. However, expression of MMP7, laminin γ2 and EGFR was not associated with infiltrative patterns, histology and mucin phenotypes (Table 2).
Association of expression among MMP-7, laminin γ2 and EGFR
We next investigated the correlations among the expression of MMP-7, laminin γ2 and EGFR. First, we investigated between MMP-7 and laminin γ2 expression. MMP-7 was often coexpressed with cytoplasmic laminin γ2 (Fig. 1g, j), but rarely coexpressed with extracellular laminin γ2 (Fig. 1h, k). MMP-7 expression was significantly more frequent with positive expression of cytoplasmic laminin γ2 than negative cases (p < 0.0001). However, positive expression of MMP-7 showed no significant correlation with expression of extracellular laminin γ2 (Table 4). We then investigated the association between laminin γ2 and EGFR expression. EGFR expression was significantly more frequent with positive expression of cytoplasmic laminin γ2 and MMP-7 than negative cases (p < 0.0001, Fig. 1i, l). No significant association between extracellular laminin γ2 and EGFR expression was detected. Hierarchical clustering of these molecules also showed virtually identical expression of MMP-7, cytoplasmic laminin γ2 and EGFR in one cluster, and that of extracellular laminin γ2 in another cluster (Fig. 2). This indicates significant associations of expression among these molecules.
Hierarchical clustering analysis of the immunohistochemical data of 790 gastric cancers to assess similarity among MMP-7, laminin γ2 and EGFR. The branch length represents the similarity between results obtained in this study. Each column represents a patient. Each row represents a marker staining as indicated on the right side. MMP-7, cytoplasmic laminin γ2 and EGFR clustered together, while extracellular laminin γ2 was in a second cluster. LNγ2 laminin-5 γ2 chain, cyto cytoplasmic pattern, extra extracellular pattern
Combined expressions of MMP-7, cytoplasmic laminin γ2 and EGFR at the invasive front and the control regions of GCs and their correlation with clinicopathologic parameters
At the invasive front of GCs, combined expressions of MMP-7, cytoplasmic laminin γ2 and EGFR were detected in 60 (8 %) of the 790 cases. At the control regions of GCs, their combined expression was detected in 5 (1 %) of the 790 cases. Combined expression at the invasive front was associated with advanced T grade (p = 0.0004), N grade (p < 0.0001) and tumor stage (p < 0.0001), whereas combined expression at the control regions was not associated with any clinicopathologic parameters (Table 5).
Discussion
In GC, various predictive factors, such as tumor size, gross appearance, cancer differentiation, depth of invasion, histological growth pattern, lymphatic invasion and venous invasion are responsible for the clinical outcomes of patients [34–40]. For several types of cancer, tumor cells at the invasive front are considered to have more aggressive behavior compared with those in the more central region [41–43] and are characterized by a dynamic process referred to as epithelial mesenchymal transition (EMT) [44, 45]. EMT is considered to be a transient and reversible process, and represents only one of the several steps required for tumor progression via invasion and metastatic spread [46], because it has also been implicated in the fundamental steps of tumorigenesis, such as invasion and metastasis [47]. In this study, we used the TMA method to examine expression of each molecule in GCs. It is well recognized that TMA is efficient for screening molecular alterations in a large number of tumor cases. However, major drawbacks of TMA analysis occur when the characteristics of sampled tissue do not always represent those of whole tumor. Although minute TMAs cannot ensure representative areas of donor specimen, we used 2-mm-diameter needles, which are large enough to evaluate the morphological appearance if representative regions are carefully selected with HE slides [48]. In terms of the possible diversity of histological components or molecular abnormalities in GCs, several previous reports have shown an excellent concordance between the results obtained from TMAs and those from full sections [49, 50]. Analyses using area-specific four-point TMAs clearly demonstrated that laminin γ2 in the invasive front largely influenced the clinical aggressiveness of colon cancer and its tendency to metastasize [51].
The present study demonstrated that MMP-7, cytoplasmic laminin γ2 and EGFR at the invasive front of GC play a pivotal role in tumor progression and regional lymph node metastasis, whereas all these molecules except cytoplasmic laminin γ2 at the control regions were not associated with any clinicopathologic parameters. In particular, cytoplasmic expression of laminin γ2 in GCs might be a potent predictive factor for tumor aggressiveness as previously reported in pancreatic ductal adenocarcinomas [52]. Laminin 5 reportedly plays an important role in EMT through down-regulation of E-cadherin and translocation of β-catenin into the nuclei [53]. Preferential expression of laminin γ2 in carcinoma cells at the invasive front and its correlation with tumor progression suggest that this molecule plays a role in the acquisition of a migrating and invading epithelial cell phenotype that is a prerequisite for malignancy [17, 23, 24]. It is known that activation of cancer-related genes in carcinoma cells affects their associated stromal cells. Certain stromal cell populations lying close to carcinoma cells may be induced to assist the invasion process by signals released by the cancer cells, stimulating the synthesis of gene products that facilitate cancer cell invasion and migration [54]. Interactions of carcinoma cells with stromal cells or with the surrounding extracellular matrix at the invasive front may result in accumulation of laminin γ2 at the invasive front. The laminin γ2 chain has been revealed to contain an epidermal growth factor (EGF)-like domain [26], and once the γ2 chain is physiologically processed by some stimulating factors such as MMP or bone morphogenetic protein-1 (BMP-1) [55, 56], the EGFR of β4 integrin would be stimulated, inducing the disruption of hemidesmosomes and tumor cell migration. The present study revealed that the combined expressions of MMP-7, laminin γ2 and EGFR at the invasive front were also associated with advanced T grade, N grade and tumor stage. However, each molecule was not significantly associated with infiltration pattern, histology and mucin phenotype. In invasive GCs, the cytoplasmic expression of laminin γ2 was reportedly detected in budding cells or dissociating cells, and its extracellular expression has been frequently detected in differentiated types [18]. There may therefore be some inconsistency between these results and the previous reports. Histologically, GCs demonstrate marked heterogeneity at both architectural and cytological levels, often with co-existence of several histologic elements. In this study, we defined the invasive front of GCs as tumor cells or clusters at the perpendicularly deepest site of tumor invasion, and punched out a 2-mm-diameter tissue core of each donor block. However, GCs containing minute amounts of tumor budding or dedifferentiation were presumably included in intestinal type GC. We also reported the significant association between the undifferentiated type of GC and N mucin phenotype [57]. However, expression of MMP7, laminin γ2 or EGFR was not associated with any mucin phenotypes. At the invasive front of GCs, meanwhile, it is suggested that aggressive GC cells with expression of these molecules do not always show tumor budding or dedifferentiation as shown in Fig. 1.
In conclusion, we clarified that expression of MMP-7, laminin γ2 or EGFR molecules, and their combinations, might be associated with tumor aggressiveness in GC. Assessment of the expression of these molecules at the invasive front of primary tumors may be clinically useful to predict the malignant behavior of GC.
References
Yasui W, Sentani K, Sakamoto N, Anami K, Naito Y, Oue N. Molecular pathology of gastric cancer: research and practice. Pathol Res Pract. 2011;207:608–12.
Hohenberger P, Gretschel S. Gastric cancer. Lancet. 2003;362:305–15.
Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–95.
Yokozaki H. Molecular characteristics of eight gastric cancer cell lines established in Japan. Pathol Int. 2000;50:767–77.
Tatematsu M, Tsukamoto T, Inada K. Stem cells and gastric cancer: role of gastric and intestinal mixed intestinal metaplasia. Cancer Sci. 2003;94:135–41.
Liotta LA. Tumor invasion and metastases–role of the extracellular matrix: rhoads memorial award lecture. Cancer Res. 1986;46:1–7.
Ii M, Yamamoto H, Adachi Y, Maruyama Y, Shinomura Y. Role of matrix metalloproteinase-7 (matrilysin) in human cancer invasion, apoptosis, growth, and angiogenesis. Exp Biol Med (Maywood). 2006;231:20–7.
Woessner JF Jr, Taplin CJ. Purification and properties of a small latent matrix metalloproteinase of the rat uterus. J Biol Chem. 1988;263:16918–25.
Miyazaki K, Hattori Y, Umenishi F, Yasumitsu H, Umeda M. Purification and characterization of extracellular matrix-degrading metalloproteinase, matrin (pump-1), secreted from human rectal carcinoma cell line. Cancer Res. 1990;50:7758–64.
Wilson CL, Matrisian LM. Matrilysin: an epithelial matrix metalloproteinase with potentially novel functions. Int J Biochem Cell Biol. 1996;28:123–36.
Yamamoto H, Adachi Y, Itoh F, Iku S, Matsuno K, Kusano M, et al. Association of matrilysin expression with recurrence and poor prognosis in human esophageal squamous cell carcinoma. Cancer Res. 1999;59:3313–6.
Adachi Y, Yamamoto H, Itoh F, Arimura Y, Nishi M, Endo T, et al. Clinicopathologic and prognostic significance of matrilysin expression at the invasive front in human colorectal cancers. Int J Cancer. 2001;95:290–4.
Liu XP, Kawauchi S, Oga A, Tsushimi K, Tsushimi M, Furuya T, et al. Prognostic significance of matrix metalloproteinase-7 (MMP-7) expression at the invasive front in gastric carcinoma. Jpn J Cancer Res. 2002;93:291–5.
Colognato H, Yurchenco PD. Form and function: the laminin family of heterotrimers. Dev Dyn. 2000;218:213–34.
Hintermann E, Quaranta V. Epithelial cell motility on laminin-5: regulation by matrix assembly, proteolysis, integrins and erbB receptors. Matrix Biol. 2004;23:75–85.
Miyazaki K. Laminin-5 (laminin-332): unique biological activity and role in tumor growth and invasion. Cancer Sci. 2006;97:91–8.
Pyke C, Romer J, Kallunki P, Lund LR, Ralfkiaer E, Dano K, et al. The gamma 2 chain of kalinin/laminin 5 is preferentially expressed in invading malignant cells in human cancers. Am J Pathol. 1994;145:782–91.
Koshikawa N, Moriyama K, Takamura H, Mizushima H, Nagashima Y, Yanoma S, et al. Overexpression of laminin gamma2 chain monomer in invading gastric carcinoma cells. Cancer Res. 1999;59:5596–601.
Fukai Y, Masuda N, Kato H, et al. Correlation between laminin-5 gamma2 chain and epidermal growth factor receptor expression in esophageal squamous cell carcinomas. Oncology. 2005;69:71–80.
Henning K, Berndt A, Katenkamp D, Kosmehl H. Loss of laminin-5 in the epithelium-stroma interface: an immunohistochemical marker of malignancy in epithelial lesions of the breast. Histopathology. 1999;34:305–9.
Masaki T, Sugiyama M, Matsuoka H, Abe N, Izumisato Y, Sakamoto A, et al. Coexpression of matrilysin and laminin-5 gamma2 chain may contribute to tumor cell migration in colorectal carcinomas. Dig Dis Sci. 2003;48:1262–7.
Okada K, Kijima H, Imaizumi T, et al. Stromal laminin-5 gamma2 chain expression is associated with the wall-invasion pattern of gallbladder adenocarcinoma. Biomed Res. 2009;30:53–62.
Yamamoto H, Kitadai Y, Yamamoto H, Oue N, Ohdan H, Yasui W, et al. Laminin gamma2 mediates Wnt5a-induced invasion of gastric cancer cells. Gastroenterology. 2009;137:242–52 (52 e1-6).
Oka T, Yamamoto H, Sasaki S, Ii M, Hizaki K, Taniguchi H, et al. Overexpression of beta3/gamma2 chains of laminin-5 and MMP7 in biliary cancer. World J Gastroenterol. 2009;15:3865–73.
Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–8.
Schenk S, Hintermann E, Bilban M, Koshikawa N, Hojilla C, Khokha R, et al. Binding to EGF receptor of a laminin-5 EGF-like fragment liberated during MMP-dependent mammary gland involution. J Cell Biol. 2003;161:197–209.
Ono Y, Nakanishi Y, Gotoh M, Sakamoto M, Hirohashi S. Epidermal growth factor receptor gene amplification is correlated with laminin-5 gamma2 chain expression in oral squamous cell carcinoma cell lines. Cancer Lett. 2002;175:197–204.
Katoh K, Nakanishi Y, Akimoto S, Yoshimura K, Takagi M, Sakamoto M, et al. Correlation between laminin-5 gamma2 chain expression and epidermal growth factor receptor expression and its clinicopathological significance in squamous cell carcinoma of the tongue. Oncology. 2002;62:318–26.
Richter P, Bohmer FD, Hindermann W, Borsi L, Hyckel P, Schleier P, et al. Analysis of activated EGFR signalling pathways and their relation to laminin-5 gamma2 chain expression in oral squamous cell carcinoma (OSCC). Histochem Cell Biol. 2005;124:151–60.
Niki T, Kohno T, Iba S, Moriya Y, Takahashi Y, Saito M, et al. Frequent co-localization of Cox-2 and laminin-5 gamma2 chain at the invasive front of early-stage lung adenocarcinomas. Am J Pathol. 2002;160:1129–41.
Sobin LH, Gospodarowicz MK. Wittekind CH eds: TNM classification of malignant tumors. 7th ed. Oxford: Wiley-Blackwell; 2009.
Lauren P. The Two Histological Main Types of Gastric Carcinoma: diffuse and So-Called Intestinal-Type Carcinoma. an Attempt at a Histo-Clinical Classification. Acta Pathol Microbiol Scand. 1965;64:31–49.
Mizoshita T, Tsukamoto T, Nakanishi H, Inada K, Ogasawara N, Joh T, et al. Expression of Cdx2 and the phenotype of advanced gastric cancers: relationship with prognosis. J Cancer Res Clin Oncol. 2003;129:727–34.
Maehara Y, Okuyama T, Moriguchi S, Orita H, Kusumoto H, Korenaga D, et al. Prophylactic lymph node dissection in patients with advanced gastric cancer promotes increased survival time. Cancer. 1992;70:392–5.
Yamao T, Shirao K, Ono H, Kondo H, Saito D, Yamaguchi H, et al. Risk factors for lymph node metastasis from intramucosal gastric carcinoma. Cancer. 1996;77:602–6.
Setala LP, Kosma VM, Marin S, Lipponen PK, Eskelinen MJ, Syrjanen KJ, et al. Prognostic factors in gastric cancer: the value of vascular invasion, mitotic rate and lymphoplasmacytic infiltration. Br J Cancer. 1996;74:766–72.
Adachi Y, Oshiro T, Mori M, Maehara Y, Sugimachi K. Tumor size as a simple prognostic indicator for gastric carcinoma. Ann Surg Oncol. 1997;4:137–40.
Kakeji Y, Maehara Y, Tomoda M, Kabashima A, Ohmori M, Oda S, et al. Long-term survival of patients with stage IV gastric carcinoma. Cancer. 1998;82:2307–11.
Hochwald SN, Brennan MF, Klimstra DS, Kim S, Karpeh MS. Is lymphadenectomy necessary for early gastric cancer? Ann Surg Oncol. 1999;6:664–70.
Saito H, Osaki T, Murakami D, Sakamoto T, Kanaji S, Oro S, et al. Macroscopic tumor size as a simple prognostic indicator in patients with gastric cancer. Am J Surg. 2006;192:296–300.
Cianchi F, Cuzzocrea S, Vinci MC, Messerini L, Comin CE, Navarra G, et al. Heterogeneous expression of cyclooxygenase-2 and inducible nitric oxide synthase within colorectal tumors: correlation with tumor angiogenesis. Dig Liver Dis. 2010;42:20–7.
Alpizar-Alpizar W, Christensen IJ, Santoni-Rugiu E, Skarstein A, Ovrebo K, Illemann M, et al. Urokinase plasminogen activator receptor on invasive cancer cells: a prognostic factor in distal gastric adenocarcinoma. Int J Cancer. 2012;131:E329–36.
Tsutsumi S, Morohashi S, Kudo Y, Akasaka H, Ogasawara H, Ono M, et al. L1 Cell adhesion molecule (L1CAM) expression at the cancer invasive front is a novel prognostic marker of pancreatic ductal adenocarcinoma. J Surg Oncol. 2011;103:669–73.
Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, et al. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci USA. 2001;98:10356–61.
Spaderna S, Schmalhofer O, Hlubek F, Berx G, Eger A, Merkel S, et al. A transient, EMT-linked loss of basement membranes indicates metastasis and poor survival in colorectal cancer. Gastroenterology. 2006;131:830–40.
Sleeman JP. The lymph node as a bridgehead in the metastatic dissemination of tumors. Recent Results Cancer Res. 2000;157:55–81.
Allan GJ, Beattie J, Flint DJ. Epithelial injury induces an innate repair mechanism linked to cellular senescence and fibrosis involving IGF-binding protein-5. J Endocrinol. 2008;199:155–64.
Sauter G, Mirlacher M. Tissue microarrays for predictive molecular pathology. J Clin Pathol. 2002;55:575–6.
Hoos A, Urist MJ, Stojadinovic A, Mastorides S, Dudas ME, Leung DH, et al. Validation of tissue microarrays for immunohistochemical profiling of cancer specimens using the example of human fibroblastic tumors. Am J Pathol. 2001;158:1245–51.
Lee HS, Cho SB, Lee HE, Kim MA, Kim JH, do Park J, et al. Protein expression profiling and molecular classification of gastric cancer by the tissue array method. Clin Cancer Res. 2007;13:4154–63.
Shinto E, Tsuda H, Ueno H, et al. Prognostic implication of laminin-5 gamma 2 chain expression in the invasive front of colorectal cancers, disclosed by area-specific four-point tissue microarrays. Lab Invest. 2005;85:257–66.
Takahashi S, Hasebe T, Oda T, et al. Cytoplasmic expression of laminin gamma2 chain correlates with postoperative hepatic metastasis and poor prognosis in patients with pancreatic ductal adenocarcinoma. Cancer. 2002;94:1894–901.
Giannelli G, Bergamini C, Fransvea E, Sgarra C, Antonaci S. Laminin-5 with transforming growth factor-beta1 induces epithelial to mesenchymal transition in hepatocellular carcinoma. Gastroenterology. 2005;129:1375–83.
Ellis V, Pyke C, Eriksen J, Solberg H, Dano K. The urokinase receptor: involvement in cell surface proteolysis and cancer invasion. Ann N Y Acad Sci. 1992;667:13–31.
Amano S, Scott IC, Takahara K, Koch M, Champliaud MF, Gerecke DR, et al. Bone morphogenetic protein 1 is an extracellular processing enzyme of the laminin 5 gamma 2 chain. J Biol Chem. 2000;275:22728–35.
Veitch DP, Nokelainen P, McGowan KA, Nguyen TT, Nguyen NE, Stephenson R, et al. Mammalian tolloid metalloproteinase, and not matrix metalloprotease 2 or membrane type 1 metalloprotease, processes laminin-5 in keratinocytes and skin. J Biol Chem. 2003;278:15661–8.
Takami H, Sentani K, Matsuda M, Oue N, Sakamoto N, Yasui W. Cytokeratin expression profiling in gastric carcinoma: clinicopathologic significance and comparison with tumor-associated molecules. Pathobiology. 2012;79:154–61.
Acknowledgments
We thank Mr. Shinichi Norimura for their excellent technical assistance and advice. This work was carried out with the kind cooperation of the Research Center for Molecular Medicine, Faculty of Medicine, Hiroshima University. We also thank the Analysis Center of Life Science, Hiroshima University, for the use of their facilities. This work was supported in part by grants-in-aid for cancer research from the Ministry of Education, Culture, Science, Sports and Technology of Japan and in part by a grant-in-aid for the Third Comprehensive 10-year Strategy for Cancer Control and for Cancer Research from the Ministry of Health, Labour and Welfare of Japan. This work was supported in part by the National Cancer Center Research and Development Fund (23-A-9).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sentani, K., Matsuda, M., Oue, N. et al. Clinicopathological significance of MMP-7, laminin γ2 and EGFR expression at the invasive front of gastric carcinoma. Gastric Cancer 17, 412–422 (2014). https://doi.org/10.1007/s10120-013-0302-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-013-0302-6