Abstract
Introduction
Bone metabolism imbalances cause bone metabolism diseases, like osteoporosis, through aging. Although some chemokines are known to be involved in bone mass regulation, many have not been investigated. Thus, the present study aimed to investigate the role of chemokine ligand 28 (CCL28) on bone metabolism.
Materials and methods
To investigate the role of CCL28 on bone metabolism, 10-week-old male wild-type and Ccl28 knockout (Ccl28 KO) mice were analyzed. Microcomputed tomography analysis and bone tissue morphometry were used to investigate the effect of Ccl28 deficiency on the bone. CCL28 localization in bone tissue was assumed by immunohistochemistry. Osteoblast and osteoclast markers were evaluated by enzyme-linked immunosorbent assay and quantitative reverse transcription-polymerase chain reaction. Finally, in vitro experiments using MC3T3-E1 and bone marrow macrophages revealed the direct effect of CCL28 on osteoblast and osteoclast.
Results
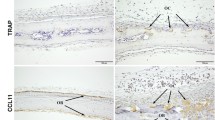
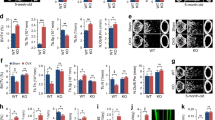
This study showed that Ccl28 deficiency significantly increased bone mass and the number of mature osteoblasts. Immunoreactivity for CCL28 was observed in osteoblasts and osteoclasts on bone tissue. Additionally, Ccl28 deficiency promoted osteoblast and osteoclast maturation. Moreover, CCL28 treatment decreased osteoblast and osteoclast activities but did not affect differentiation.
Conclusion
In summary, this study indicated that CCL28 is one of the negative regulators of bone mass by suppressing osteoblast and osteoclast activities. These results provide important insights into bone immunology and the selection of new osteoporosis treatments.







Similar content being viewed by others
References
Hadjidakis DJ, Androulakis II (2006) Bone remodeling. Ann N Y Acad Sci 1092:385–396
Walsh JS (2015) Normal bone physiology, remodeling, and its hormonal regulation. Surgery (United Kingdom) 33:1–6
Zlotnik A, Yoshie O (2012) The chemokine superfamily revisited. Immunity 36:705–716
Liu YZ, Dvornyk V, Lu Y, Shen H, Lappe JM, Recker RR, Deng HW (2005) A novel pathophysiological mechanism for osteoporosis suggested by an in vivo gene expression study of circulating monocytes. J Biol Chem 280:29011–29016
Mohan S, Hu Y, Edderkaoui B (2019) Chemokine receptor 3 is a negative regulator of trabecular bone mass in female mice. J Cell Biochem 120:13974–13984
Wintges K, Beil FT, Albers J, Jeschke A, Schweizer M, Claass B, Tiegs G, Amling M, Schinke T (2013) Impaired bone formation and increased osteoclastogenesis in mice lacking chemokine (C-C motif) ligand 5 (Ccl5). J Bone Miner Res 28:2070–2080
Kindstedt E, Holm CK, Sulniute R, Martinez-Carrasco I, Lundmark R, Lundberg P (2017) CCL11, a novel mediator of inflammatory bone resorption. Sci Rep 7:1–10
Liu B, Wilson E (2010) The antimicrobial activity of CCL28 is dependent on C-terminal positively-charged amino acids. Eur J Immunol 40:186–196
Pan J, Kunkel EJ, Gosslar U, Lazarus N, Langdon P, Broadwell K, Vierra MA, Genovese MC, Butcher EC, Soler D (2000) Cutting edge: a novel chemokine ligand for CCR10 and CCR3 expressed by epithelial cells in mucosal tissues. J Immunol 165:2943–2949
Hieshima K, Ohtani H, Shibano M, Izawa D, Nakayama T, Kawasaki Y, Shiba F, Shiota M, Katou F, Saito T, Yoshie O (2003) CCL28 has dual roles in mucosal immunity as a chemokine with broad-spectrum antimicrobial activity. J Immunol 170:1452–1461
Hieshima K, Kawasaki Y, Hanamoto H, Nakayama T, Nagakubo D, Kanamaru A, Yoshie O (2004) CC chemokine ligands 25 and 28 play essential roles in intestinal extravasation of IgA antibody-secreting cells. J Immunol 2004:3668–3675
Matsuo K, Nagakubo D, Yamamoto S, Shigeta A, Tomida S, Fujita M, Hirata T, Tsunoda I, Nakayama T, Yoshie O (2018) CCL28-deficient mice have reduced IgA antibody-secreting cells and an altered microbiota in the colon. J Immunol 200:800–809
Yoshimi K, Kunihiro Y, Kaneko T, Nagahora H, Voigt B, Mashimo T (2016) SsODN-mediated knock-in with CRISPR-Cas for large genomic regions in zygotes. Nat Commun 7:2–11
Hosoya A, Takahama A, Nakamura H (2017) Localization of RELM-β/FIZZ2 is associated with cementum formation. Anat Rec (Hoboken) 300:1865–1874
Yukita A, Hosoya A, Ito Y, Katagiri T, Asashima M, Nakamura H (2012) Ubc9 negatively regulates BMP-mediated osteoblastic differentiation in cultured cells. Bone 50:1092–1099
Nagafusa H, Sayama K (2020) Age-related chemokine alterations affect IgA secretion and gut immunity in female mice. Biogerontology 21:609–618
Niwa H, Yamamura K, Miyazaki J (1991) Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193–199
Yogo K, Mizutamari M, Mishima K, Takenouchi H, Ishida-Kitagawa N, Sasaki T, Takeya T (2006) Src homology 2 (SH2)-containing 5′-inositol phosphatase localizes to podosomes, and the SH2 domain is implicated in the attenuation of bone resorption in osteoclasts. Endocrinology 147:3307–3317
Patti A, Gennari L, Merlotti D, Dotta F, Nuti R (2013) Endocrine actions of osteocalcin. Int J Endocrinol 2013:846480
Xian L, Wu X, Pang L, Lou M, Rosen CJ, Qiu T, Crane J, Frassica F, Zhang L, Rodriguez JP, Jia X, Yakar S, Xuan S, Efstratiadis A, Wan M, Cao X (2012) Matrix IGF1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat Med 18:1095–1101
Kim N, Kadono Y, Takami M, Lee J, Lee SH, Okada F, Jung HK, Kobayashi T, Odgren PR, Nakano H, Yeh WC, Lee SK, Lorenzo JA, Choi Y (2005) Osteoclast differentiation independent of the TRANCE-RANK-TRAF6 axis. J Exp Med 202:589–595
Udagawa N, Kotake S, Kamatani N, Takahashi N, Suda T (2002) The molecular mechanism of osteoclastogenesis in rheumatoid arthritis. Arthritis Res 4:281–289
Shiratori T, Kyumoto-Nakamura Y, Kukita A, Uehara N, Zhang J, Koda K, Kamiya M, Badawy T, Tomoda E, Xu X, Yamaza T, Urano Y, Koyano K, Kukita T (2018) IL-1β induces pathologically activated osteoclasts bearing extremely high levels of resorbing activity: a possible pathological subpopulation of osteoclasts, accompanied by suppressed expression of kindlin-3 and talin-1. J Immunol 200:218–228
Soysa NS, Alles N, Aoki K, Ohya K (2012) Osteoclast formation and differentiation. An overview. J Med Dent Sci 59:65–74
Dean DD, Schwartz Z, Bonewald L, Muniz OE, Morales S, Gomez R, Brooks BP, Qiao M, Howell DS, Boyan BD (1994) Matrix vesicles produced by osteoblast-like cells in culture become significantly enriched in proteoglycan-degrading metalloproteinases after addition of beta-glycerophosphate and ascorbic acid. Calcif Tissue Int 54:399–408
Mukherjee A, Rotwein P (2009) Akt promotes BMP2-mediated osteoblast differentiation and bone development. J Cell Sci 122:716–726
Brylka LJ, Schinke T (2019) Chemokines in physiological and pathological bone remodeling. Front Immunol 10:1–19
Chen Z, Kim SJ, Essani AB, Volin MV, Vila OM, Swedler W, Arami S, Volkov S, Sardin LV, Sweiss N, Shahrara S (2015) Characterising the expression and function of CCL28 and its corresponding receptor, CCR10, in RA pathogenesis. Ann Rheum Dis 74:1898–1906
Hoshino A, Iimura T, Ueha S, Hanada S, Maruoka Y, Mayahara M, Suzuki K, Imai T, Ito M, Manome Y, Yasuhara M, Kirino T, Yamaguchi A, Matsushima K, Yamamoto K (2010) The deficiency of chemokine receptor CCR1 causes osteopenia due to impaired functions of osteoclasts and osteoblasts. J Biol Chem 285:28826–28837
Lee JW, Hoshino A, Inoue K, Saitou T, Uehara S, Kobayashi Y, Ueha S, Matsushima K, Yamaguchi A, Imai Y, Iimura T (2017) The HIV co-receptor CCR5 regulates osteoclast function. Nat Commun 8:1–16
Li JY, Chassaing B, Tyagi AM, Vaccaro C, Luo T, Adams J, Darby TM, Weitzmann MN, Mulle JG, Gewirtz AT, Jones RM, Pacifici R (2016) Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Investig 126:2049–2063
Novince CM, Whittow CR, Aartun JD, Hathaway JD, Poulides N, Chavez MB, Steinkamp HM, Kirkwood KA, Huang E, Westwater C, Kirkwood KL (2017) Commensal gut microbiota immunomodulatory actions in bone marrow and liver have catabolic effects on skeletal homeostasis in health. Sci Rep 7:1–17
Acknowledgements
The authors thank Dr. Keiichiro Yogo (Shizuoka University) for providing the NIH3T3 cells that stably expressed the human M-CSF vector and Dr. Tomohisa Ishikawa and Dr. Momoka Yamaguchi (University of Shizuoka) for providing the antibodies. Moreover, Mr. Fumiya Kamiya, Kurumi Maeda, Hirofumi Fukazawa, Ayano Hashimoto, and Sano Takayuki (Shizuoka University) are also thanked for their technical assistance. This work was supported, in part, by a grant-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology (16K12720).
Author information
Authors and Affiliations
Contributions
RI, TT, KS, and AY contributed to the conception and design of the research. RI, TT, KY, YI, TK, MH, and TN performed the experiments and analyzed the data. RI, TT, NH, KS, and AY interpreted the results. RI and TT prepared the figures. RI drafted the manuscript, and HN, KS, and AY edited and revised the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
774_2021_1210_MOESM1_ESM.tiff
Supplementary file1 Fig. S1 genotyping in a conventional PCR with specific primers and sequence. a Ccl28 gene knockout target region. b Sequence results confirmed that the Ccl28 gene was mutated by a 13-base deletion. c Primer and amplification regions used for genotyping of mice used in the experiment. d Primer1 and primer2 can amplify DNA with WT and Ccl28 KO, respectively. Ccl28 KO reduces the amplification size by 13 bases because primer3 can amplify the region containing the defective site. PCR results confirmed that the mice used were Ccl28 KO. (TIFF 185 KB)
774_2021_1210_MOESM2_ESM.tiff
Supplementary file2 Fig. S2. Effect of genotype and treatment on organ weights in male and female animals. The animals were sacrificed and the a body, b kidney, c liver, d colon, e ileum, f caecal, g spleen, and h thymus weights were measured. Data are presented as mean ± SEM; **P < 0.01, *****P < 0.00001 vs WT. (TIFF 114 KB)
774_2021_1210_MOESM3_ESM.tiff
Supplementary file3 Fig. S3. The expressions of Ccl28 and Ccr3 in MC3T3-E1. a, b The relative expression levels of the indicated genes were measured by qRT-PCR in MC3T3-E1 treated with αMEM or OMM (a Ccl28, b Ccr3). These markers were normalized to Gapdh expression. Data are presented as mean ± SE, n = 3; *P < 0.05 vs αMEM. (TIFF 65 KB)
774_2021_1210_MOESM4_ESM.tiff
Supplementary file4 Fig. S4. The expressions of Ccr1 and Il-6 in the bones of 10-week-old WT and Ccl28 KO mice. a The relative expression levels of Ccr1 was measured by qRT-PCR in the bones of 10-week-old WT and Ccl28 KO mice. b The relative expression levels of Il-6 were measured by QRT-PCR in the livers of 10-week-old WT and Ccl28 KO mice. These markers were normalized to Gapdh expression. Data are presented as mean ± SE, n = 3; *P < 0.05 vs WT mice. (TIFF 64 KB)
774_2021_1210_MOESM5_ESM.tiff
Supplementary file5 Fig. S5. Changes in CCL28, CCR3, and CCR10 expressions with aging. a–c The relative expression levels of the indicated genes were measured by qRT-PCR in 10-week-old (young) and 84-week-old (aged) mice bone (a Ccl28, b Ccr3, and c Ccr10). These markers were normalized to Gapdh expression. Data are presented as mean ± SE, n = 3; *P < 0.05, ***P < 0.001, *****P < 0.00001 vs young group (TIFF 26370 KB)
About this article
Cite this article
Iwamoto, R., Takahashi, T., Yoshimi, K. et al. Chemokine ligand 28 (CCL28) negatively regulates trabecular bone mass by suppressing osteoblast and osteoclast activities. J Bone Miner Metab 39, 558–571 (2021). https://doi.org/10.1007/s00774-021-01210-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-021-01210-9