Abstract
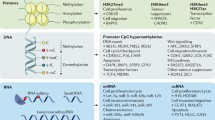
Knowledge about the molecular profile of tumor tissues is crucial to effectively target cancer cells, because cancer is a genetic disease that involves multiple genetic and epigenetic alterations. Prominent aberrations include gene mutation, amplification, loss or deletion, as well as epigenetic alterations of the promoter DNA CpG islands. All of these aberrations can lead to dynamic changes in cancer cells, as demonstrated using resected tumor samples. There are two distinct pathological types of gastric cancer: the diffuse type and the intestinal type of gastric cancer. Diffuse type gastric cancer harbors aberrations in the FGFR2/ErbB3/PI3 kinase pathway, while intestinal type gastric cancer has an activated ErbB2 oncogenic pathway. On the other hand, the prometastatic oncogene PRL-3 is commonly activated in both types of advanced gastric cancer, and might represent a relevant therapeutic target for gastric cancer with lymph node metastasis or peritoneal dissemination. Numerous tumor suppressor genes can inhibit such oncogenic pathways, and DNA methylation in CpG islands of gene promoters is frequently found to suppress the expression of such genes in gastric cancer. Helicobacter pylori infection in normal gastric mucosa may cause p53 mutations through activation of activation-induced cytidine deaminase (AID) and/or promoter DNA methylation of E-cadherin, an initiator of gastric cancer, and such abnormalities are found even in the precancerous stage of gastric carcinogenesis. In addition, it has been demonstrated that there are highly relevant methylation genes involved in cancer (HRMGs) that exhibit very frequent cancer-specific methylation in gastric cancer. Such genes are potential targets for cancer treatment, and might also serve as biomarkers of gastric cancer for either the diagnosis or for determining the prognosis or the response to treatment.
Similar content being viewed by others
References
Wu CW, Hsiung CA, Lo SS, Hsieh MC, Chen JH, Li AF, et al. Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol 2006;7:309–315.
Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, et al.; Japan Clinical Oncology Group. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med 2008;359:453–462.
Hu JK, Yang K, Zhang B, Chen XZ, Chen ZX, Chen JP. D2 plus para-aortic lymphadenectomy versus standardized D2 lymphadenectomy in gastric cancer surgery. Surg Today 2009;39:207–213.
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74–108.
Pera M, Manterola C, Vidal O, et al. Epidemiology of esophageal adenocarcinoma. J Surg Oncol 2005;92:151–159.
Yamashita K, Sakuramoto S, Katada N, et al. Diffuse type advanced gastric cancer showing dismal prognosis is characterized by deeper invasion and emerging peritoneal cancer cell: the latest comparative study to intestinal advanced gastric cancer. Hepatogastroenterology 2009;56:276–281.
Kountouras J, Zavos C, Chatzopoulos D, et al. New aspects of Helicobacter pylori infection involvement in gastric oncogenesis. J Surg Res 2008;146:149–158.
Japanese Gastric Cancer Association Registration Committee, Maruyama K, Kaminishi M, Hayashi K, et al. Gastric cancer treated in 1991 in Japan: data analysis of nationwide registry. Gastric Cancer 2006;9:51–66.
Henson DE, Dittus C, Younes M, et al. Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973–2000: increase in the signet ring cell type. Arch Pathol Lab Med 2004;128:765–770.
Lauwers GY. Defining the pathologic diagnosis of metaplasia, atrophy, dysplasia, and gastric adenocarcinoma. J Clin Gastroenterol 2003;36:S37–S43.
Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell 1996;87:159–170.
Kikuchi S, Hiki Y, Tsutsumi O, Kobayashi N, Tsukamoto H, Shimao H, et al. Surgical outcome of curative resection in patients with Borrmann type IV gastric carcinoma with particular reference to the extent of lymph node metastasis. Hepatogastroenterology 2000;47:890–892.
Ikeguchi M, Miyake T, Matsunaga T, Yamamoto M, Fukumoto Y, Yamada Y, et al. Recent results of therapy for scirrhous gastric cancer. Surg Today 2009;39:290–294.
Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 2007;357:1810–1820.
Emi Y, Yamamoto M, Takahashi I, Orita H, Kakeji Y, Kohnoe S, et al. Phase II study of weekly paclitaxel by one-hour infusion for advanced gastric cancer. Surg Today 2008;38:1013–1020.
Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 2008;9(3):215–221.
Ikeguchi M, Miyake T, Matsunaga T, Yamamoto M, Fukumoto Y, Yamada Y, et al. Recent results of therapy for scirrhous gastric cancer. Surg Today 2009;39(4):290–294.
Hattori Y, Odagiri H, Nakatani H, et al. K-sam, an amplified gene in stomach cancer, is a member of the heparin-binding growth factor receptor genes. Proc Natl Acad Sci USA 1990;87:5983–5987.
Nakatani H, Sakamoto H, Yoshida T, et al. Isolation of an amplified DNA sequence in stomach cancer. Jpn J Cancer Res 1990;81:707–710.
Miki T, Fleming TP, Bottaro DP, et al. Expression cDNA cloning of the KGF receptor by creation of a transforming autocrine loop. Science 1991;251:72–75.
Hattori Y, Itoh H, Uchino S, et al. Immunohistochemical detection of K-sam protein in stomach cancer. Clin Cancer Res 1996;2:1373–1381.
Jang JH, Shin KH, Park JG. Mutations in fibroblast growth factor receptor 2 and fibroblast growth factor receptor 3 genes associated with human gastric and colorectal cancers. Cancer Res 2001;61:3541–3543.
Yashiro M, Shinto O, Nakamura K, et al. Synergistic antitumor effects of FGFR2 inhibitor with 5-fluorouracil on scirrhous gastric carcinoma. Int J Cancer 2010;126:1004–1016.
Cantley LC AK, Carpenter C, Duckworth B, Graziani A, Kapeller R, et al. Oncogenes and signal transduction. Cell 1991;64:281–302.
Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science 2004;304:554.
Byun DS, Cho K, Ryu BK, et al. Frequent monoallelic deletion of PTEN and its reciprocal association with PIK3CA amplification in gastric carcinoma. Int J Cancer 2003;104:318–327.
Kobayashi M, Nagata S, Iwasaki T, et al. Dedifferentiation of adenocarcinomas by activation of phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA 1999;96:4874–4879.
Kobayashi M, Iwamatsu A, Shinohara-Kanda A, et al. Activation of ErbB3-PI3-kinase pathway is correlated with malignant phenotypes of adenocarcinomas. Oncogene 2003;22:1294–1301.
Carraway KL III, Sliwkowski MX, Platko JV, et al. The erbB3 gene product is a receptor for heregulin. J Biol Chem 1994;269:14303–14306.
Zhang XL, Yang YS, Xu DP, et al. Comparative study on overexpression of HER2/neu and HER3 in gastric cancer. World J Gastroenterol 2009;33:2112–2118.
Kunii K, Davis L, Gorenstein J, et al. FGFR2-amplified gastric cancer cell lines require FGFR2 and Erbb3 signaling for growth and survival. Cancer Res 2008;68:2340–2348.
Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer 2004;4:118–132.
Becker KF, Atkinson MJ, Reich U, et al. E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res 1994;54:3845–3852.
Tamura G, Sakata K, Nishizuka S, et al. Inactivation of the E-cadherin gene in primary gastric carcinomas and gastric carcinoma cell lines. Jpn J Cancer Res 1996;87:1153–1159.
Machado JC, Oliveira C, Carvalho R, et al. E-cadherin gene (CDH1) promoter methylation as the second hit in sporadic diffuse gastric carcinoma. Oncogene 2001;20:1525–1528.
Tamura G, Yin J, Wang S, et al. E-Cadherin gene promoter hypermethylation in primary human gastric carcinomas. J Natl Cancer Inst 2000;92:569–573.
Graziano F, Arduini F, Ruzzo A, et al. E-cadherin gene promoter hypermethylation in patients with surgically resected, nodepositive, diffuse gastric cancer. Clin Cancer Res 2004;10:2784–2789.
Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature 1998;392(6672):190–193.
Guilford P, Hopkins J, Harraway J, et al. E-cadherin germline mutations in familial gastric cancer. Nature 1998;392:402–405.
Humar B, Guilford P. Hereditary diffuse gastric cancer: a manifestation of lost cell polarity. Cancer Sc 2009;100:1151–1157.
Humar B, Blair V, Charlton A, et al. E-cadherin deficiency initiates gastric signet-ring cell carcinoma in mice and man. Cancer Res 2009;69:2050–2056.
Liu YC, Shen CY, Wu HS, et al. Mechanisms inactivating the gene for E-cadherin in sporadic gastric carcinomas. World J Gastroenterol 2006;12:2168–2173.
Zheng ZH, Sun XJ, Zhou HT, et al. Analysis of metastasis suppressing function of E-cadherin in gastric cancer cells by RNAi. World J Gastroenterol 2005;11:2000–2003.
Wong AS, Gumbiner BM. Adhesion-independent mechanism for suppression of tumor cell invasion by E-cadherin. J Cell Biol 2003;161:1191–1203.
Asaka M, Kimura T, Kato M, et al. Possible role of Helicobacter pylori infection in early gastric cancer development. Cancer 1994;73:2691–2694.
El-Omar EM, Carrington M, Chow WH, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 2000;404:398–402.
Qian X, Huang C, Cho CH, et al. E-cadherin promoter hypermethylation induced by interleukin-1beta treatment or H. pylori infection in human gastric cancer cell lines. Cancer Lett 2008;263:107–113.
Tanner M, Hollmen M, Junttila TT, et al. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol 2005;16:273–278.
Alvarado D, Klein DE, Lemmon MA. ErbB2 resembles an autoinhibited invertebrate epidermal growth factor receptor. Nature 2009;461:287–291.
Yano T, Doi T, Ohtsu A, et al. Comparison of HER2 gene amplification assessed by fluorescence in situ hybridization and HER2 protein expression assessed by immunohistochemistry in gastric cancer. Oncol Rep 2006;15:65–71.
Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol 2008;19:1523–1529.
Matsui Y, Inomata M, Tojigamori M, et al. Suppression of tumor growth in human gastric cancer with HER2 overexpression by an anti-HER2 antibody in a murine model. Int J Oncol 2005;27:681–685.
Fujimoto-Ouchi K, Sekiguchi F, Yasuno H, et al. Antitumor activity of trastuzumab in combination with chemotherapy in human gastric cancer xenograft models. Cancer Chemother Pharmacol 2007;59:795–805.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376(9742):687–697. Epub 2010 Aug 19. Erratum in: Lancet 2010;376(9749):1302.
Vogelstein B, and Kinzler KW. Cancer genes and the pathways they control. Nat Med 2004;10:789–799.
Fenoglio-Preiser CM, Wang J, Stemmermann GN, et al. TP53 and gastric carcinoma: a review. Hum Mutat 2003;21:258–270.
Huang JQ, Zheng GF, Sumanac K, et al. Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology 2003;125:1636–1644.
Matsumoto Y, Marusawa H, Kinoshita K, et al. Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat Med 2007;13:470–476.
Shiao YH, Rugge M, Correa P, et al. p53 alteration in gastric precancerous lesions. Am J Pathol 1994;144:511–517.
Horii A, Nakatsuru S, Miyoshi Y, et al. The APC gene, responsible for familial adenomatous polyposis, is mutated in human gastric cancer. Cancer Res 1992;52:3231–3233.
Abraham SC, Nobukawa B, Giardiello FM, et al. Sporadic fundic gland polyps: common gastric polyps arising through activating mutations in the beta-catenin gene. Am J Pathol 2001;158:1005–1010.
Ebert MP, Fei G, Kahmann S, et al. Increased beta-catenin mRNA levels and mutational alterations of the APC and betacatenin gene are present in intestinal-type gastric cancer. Carcinogenesis 2002;23:87–91.
Shirasawa S, FM, Yokoyama N, Sasazuki T. Altered growth of human colon cancer cell lines disrupted at activated Ki-ras. Science 1993;260:85–88.
Rak J, Mitsuhashi Y, Bayko L, Filmus J, Shirasawa S, Sasazuki T, et al. Mutant ras oncogenes upregulate VEGF/VPF expression: implications for induction and inhibition of tumor angiogenesis. Cancer Res 1995;55:4575–4580.
Kusano M, Toyota M, Suzuki H, et al. Genetic, epigenetic, and clinicopathologic features of gastric carcinomas with the CpG island methylator phenotype and an association with Epstein-Barr virus. Cancer 2006;106:1467–1479.
Saha S, Bardelli A, Buckhaults P, et al. A phosphatase associated with metastasis of colorectal cancer. A phosphatase associated with metastasis of colorectal cancer. Science 2001;294:1343–1346.
Bardelli A, Saha S, Sager JA, et al. PRL-3 expression in metastatic cancers. Clin Cancer Res 2003;9:5607–5615.
Miskad UA, Semba S, Kato H, et al. Expression of PRL-3 phosphatase in human gastric carcinomas: close correlation with invasion and metastasis. Pathobiology 2004;71:176–184.
Miskad UA, Semba S, Kato H, et al. High PRL-3 expression in human gastric cancer is a marker of metastasis and grades of malignancies: an in situ hybridization study. Virchows Arch 2007;450:303–310.
Li ZR, Wang Z, Zhu BH, et al. Association of tyrosine PRL-3 phosphatase protein expression with peritoneal metastasis of gastric carcinoma and prognosis. Surg Today 2007;37:646–651.
Wang Z, He YL, Cai SR, et al. Expression and prognostic impact of PRL-3 in lymph node metastasis of gastric cancer: its molecular mechanism was investigated using artificial microRNA interference. Int J Cancer 2008;123:1439–1447.
Hatate K, Yamashita K, Hirai K, et al. Liver metastasis of colorectal cancer by protein-tyrosine phosphatase type 4A, 3 (PRL-3) is mediated through lymph node metastasis and elevated serum tumor markers such as CEA and CA19-9. Oncol Rep 2008;20:737–743.
Wang Z, Cai SR, He YL, et al. High expression of PRL-3 can promote growth of gastric cancer and exhibits a poor prognostic impact on patients. Ann Surg Oncol 2009;16:208–219.
Wang Z, Cai SR, He YL, et al. Elevated PRL-3 expression was more frequently detected in the large primary gastric cancer and exhibits a poor prognostic impact on the patients. J Cancer Res Clin Oncol 2009;135:1041–1046.
Dai N, Lu AP, Shou CC, et al. Expression of phosphatase regenerating liver 3 is an independent prognostic indicator for gastric cancer. World J Gastroenterol. 2009;15:1499–1505.
Ooki A, Yamashita K, Kikuchi S, et al. Phosphatase of regenerating liver-3 as a prognostic biomarker in histologically nodenegative gastric cancer. Oncol Rep 2009;21:1467–1475.
Ooki A, Yamashita K, Kikuchi S, Sakuramoto S, Katada N, Watanabe M. Phosphatase of regenerating liver-3 as a convergent therapeutic target for lymph node metastasis in esophageal squamous cell carcinoma. Int J Cancer 2010;127(3):543–554.
Zeng Q, Dong JM, Guo K, et al. PRL-3 and PRL-1 promote cell migration, invasion, and metastasis. Cancer Res 2003;63:2716–2722.
Wu X, Zeng H, Zhang X, et al. Phosphatase of regenerating liver-3 promotes motility and metastasis of mouse melanoma cells. Am J Pathol 2004;164:2039–2054.
Guo K, Li J, Wang H, et al. PRL-3 initiates tumor angiogenesis by recruiting endothelial cells in vitro and in vivo. Cancer Res 2006;66:9625–9635.
Peng L, Jin G, Wang L, et al. Identification of integrin alpha1 as an interacting protein of protein tyrosine phosphatase PRL-3. Biochem Biophys Res Commun 2006;342:179–183.
Peng L, Xing X, Li W, et al. PRL-3 promotes the motility, invasion, and metastasis of LoVo colon cancer cells through PRL-3-integrin beta1-ERK1/2 and-MMP2 signaling. Mol Cancer 8:1–13.
Forte E, Orsatti L, Talamo F, et al. Ezrin is a specific and direct target of protein tyrosine phosphatase PRL-3. Biochim Biophys Acta 2008;1783:334–344.
Liu Y, Zhou J, Chen J, et al. PRL-3 promotes epithelial mesenchymal transition by regulating cadherin directly. Cancer Biol Ther 2009;8:1352–1359.
Toyota M, Ahuja N, Ohe-Toyota M, et al. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci USA 1999;96:8681–8686.
Kim MS, Lee J, Sidransky D. DNA methylation markers in colorectal cancer. Cancer Metastasis Rev 2010;29:181–206.
Yamashita K, Upadhyay S, Osada M, et al. Pharmacologic unmasking of epigenetically silenced tumor suppressor genes in esophageal squamous cell carcinoma. Cancer Cell 2002;2:485–495.
Tokumaru Y, Yamashita K, Osada M, et al. Inverse correlation between cyclin A1 hypermethylation and p53 mutation in head and neck cancer identified by reversal of epigenetic silencing. Cancer Res 2004;64:5982–5987.
Mandelker DL, Yamashita K, Tokumaru Y, et al. PGP9.5 promoter methylation is an independent prognostic factor for esophageal squamous cell carcinoma. Cancer Res 2005;65:4963–4968.
Kim MS, Yamashita K, Baek JH, et al. N-methyl-D-aspartate receptor type 2B is epigenetically inactivated and exhibits tumorsuppressive activity in human esophageal cancer. Cancer Res 2006;66:3409–3418.
Yamashita K, Park HL, Kim MS, et al. PGP9.5 methylation in diffuse-type gastric cancer. Cancer Res 2006;66:3921–3927.
Liu JW, Kim MS, Nagpal J, et al. Quantitative hypermethylation of NMDAR2B in human gastric cancer. Int J Cancer 2007;121:1994–2000.
Kim MS, Chang X, Nagpal JK, et al. The N-methyl-D-aspartate receptor type 2A is frequently methylated in human colorectal carcinoma and suppresses cell growth. Oncogene 2008;27:2045–2054.
Kim MS, Yamashita K, Chae YK, et al. A promoter methylation pattern in the N-methyl-D-aspartate receptor 2B gene predicts poor prognosis in esophageal squamous cell carcinoma. Clin Cancer Res 2007;13:6658–6665.
Kim MS, Chang X, Yamashita K, et al. Aberrant promoter methylation and tumor suppressive activity of the DFNA5 gene in colorectal carcinoma. Oncogene 2008;27:3624–3634.
Yamashita K, Kim MS, Park HL, et al. HOP/OB1/NECC1 promoter DNA is frequently hypermethylated and involved in tumorigenic ability in esophageal squamous cell carcinoma. Mol Cancer Res 2008;6:31–41.
Park HL, Kim MS, Yamashita K, et al. DCC promoter hypermethylation in esophageal squamous cell carcinoma. Int J Cancer 2008;122:2498–2502.
Kim MS, Lebron C, Nagpal JK, et al. Methylation of the DFNA5 increases risk of lymph node metastasis in human breast cancer. Biochem Biophys Res Commun 2008;370:38–43.
Hoque MO, Kim MS, Ostrow KL, et al. Genome-wide promoter analysis uncovers portions of the cancer methylome. Cancer Res 2008;68:2661–2670.
Tokumaru Y, Yamashita K, Kim MS, et al. The role of PGP9.5 as a tumor suppressor gene in human cancer. Int J Cancer 2008;123:753–759.
Kim MS, Louwagie J, Carvalho B, et al. Promoter DNA methylation of oncostatin m receptor-beta as a novel diagnostic and therapeutic marker in colon cancer. PLoS One 2009;4:e6555.
Kim MS, Chang X, LeBron C, et al. Neurofilament heavy polypeptide regulates the Akt-beta-catenin pathway in human esophageal squamous cell carcinoma. PLoS One 2010;5:e9003.
Ooki A, Yamashita K, Kikuchi S, et al. Potential utility of HOP homeobox gene promoter methylation as a marker of tumor aggressiveness in gastric cancer. Oncogene 2010;29(22):3263–3275.
Chen RZ, Pettersson U, Beard C, et al. DNA hypomethylation leads to elevated mutation rates. Nature 1998;395:89–93.
De Smet C, Loriot A. DNA hypomethylation in cancer: epigenetic scars of a neoplastic journey. Epigenetics 2010;5(3):206–213.
Leung SY, Yuen ST, Chung LP, et al. hMLH1 promoter methylation and lack of hMLH1 expression in sporadic gastric carcinomas with high-frequency microsatellite instability. Cancer Res 1999;59:159–164.
Fleisher AS, Esteller M, Wang S, et al. Hypermethylation of the hMLH1 gene promoter in human gastric cancers with microsatellite instability. Cancer Res 1999;59:1090–1095.
Grady WM, Willis J, Guilford PJ, et al. Methylation of the CDH1 promoter as the second genetic hit in hereditary diffuse gastric cancer. Nat Genet 2000;26:16–17.
Tsuchiya T, Tamura G, Sato K, et al. Distinct methylation patterns of two APC gene promoters in normal and cancerous gastric epithelia. Oncogene 2000;19:3642–3646.
Kawakami K, Brabender J, Lord RV, et al. Hypermethylated APC DNA in plasma and prognosis of patients with esophageal adenocarcinoma. J Natl Cancer Inst 2000;92:1805–1811.
Lee YY, Kang SH, Seo JY, et al. Alterations of p16INK4A and p15INK4B genes in gastric carcinomas. Cancer 1997;80:1889–1896.
Suzuki H, Itoh F, Toyota M, et al. Distinct methylation pattern and microsatellite instability in sporadic gastric cancer. Int J Cancer 1999;83:309–313.
Iida S, Akiyama Y, Nakajima T, et al. Alterations and hypermethylation of the p14(ARF) gene in gastric cancer. Int J Cancer 2000;87:654–658.
To KF, Leung WK, Lee TL, et al. Promoter hypermethylation of tumor-related genes in gastric intestinal metaplasia of patients with and without gastric cancer. Int J Cancer 2002;102:623–628.
Leung WK, Yu J, Ng EK, et al. Concurrent hypermethylation of multiple tumor-related genes in gastric carcinoma and adjacent normal tissues. Cancer 2001;91:2294–2301.
Endoh Y, Tamura G, Ajioka Y, et al. Frequent hypermethylation of the hMLH1 gene promoter in differentiated-type tumors of the stomach with the gastric foveolar phenotype. Am J Pathol 2000;157:717–722.
Kang GH, Shim YH, Jung HY, et al. CpG island methylation in premalignant stages of gastric carcinoma. Cancer Res 2001;61:2847–2851.
Waki T, Tamura G, Tsuchiya T, et al. Promoter methylation status of E-cadherin, hMLH1, and p16 genes in nonneoplastic gastric epithelia. Am J Pathol 2002;161:399–403.
Suzuki K, Suzuki I, Leodolter A, et al. Global DNA demethylation in gastrointestinal cancer is age dependent and precedes genomic damage. Cancer Cell 2006;9:199–207.
Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA 1996;93:9821–9826.
Li M, Chen WD, Papadopoulos N, Goodman SN, Bjerregaard NC, Laurberg S, et al. Sensitive digital quantification of DNA methylation in clinical samples. Nat Biotechnol 2009;27:858–863.
Kanai Y, Ushijima S, Kondo Y, et al. DNA methyltransferase expression and DNA methylation of CPG islands and pericentromeric satellite regions in human colorectal and stomach cancers. Int J Cancer 2001;91:205–212.
Kang SH, Choi HH, Kim SG, et al. Transcriptional inactivation of the tissue inhibitor of metalloproteinase-3 gene by DNA hypermethylation of the 5′-CpG island in human gastric cancer cell lines. Int J Cancer 2000;86:632–635.
Li QL, Ito K, Sakakura C, et al. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell 2002;109:113–124.
Waki T, Tamura G, Sato M, et al. Promoter methylation status of DAP-kinase and RUNX3 genes in neoplastic and non-neoplastic gastric epithelia. Cancer Sci 2003;94:360–364.
Satoh A, Toyota M, Itoh F, et al. DNA methylation and histone deacetylation associated with silencing DAP kinase gene expression in colorectal and gastric cancers. Br J Cancer 2002;86:1817–1823.
Kang GH, Lee S, Kim WH, et al. Epstein-Barr virus-positive gastric carcinoma demonstrates frequent aberrant methylation of multiple genes and constitutes CpG island methylator phenotype-positive gastric carcinoma. Am J Pathol 2002;160:787–794.
Lee TL, Leung WK, Chan MW, et al. Detection of gene promoter hypermethylation in the tumor and serum of patients with gastric carcinoma. Clin Cancer Res 2002;8:1761–1766.
Oue N, Shigeishi H, Kuniyasu H, et al. Promoter hypermethylation of MGMT is associated with protein loss in gastric carcinoma. Int J Cancer 2001;93:805–809.
Park TJ, Han SU, Cho YK, et al. Methylation of O(6)-methylguanine-DNA methyltransferase gene is associated significantly with K-ras mutation, lymph node invasion, tumor staging, and disease free survival in patients with gastric carcinoma. Cancer 2001;92:2760–2768.
Hayashi K, Yokozaki H, Goodison S, et al. Inactivation of retinoic acid receptor beta by promoter CpG hypermethylation in gastric cancer. Differentiation 2001;68:13–21.
Oue N, Motoshita J, Yokozaki H, et al. Distinct promoter hypermethylation of p16INK4a, CDH1, and RAR-beta in intestinal, diffuse-adherent, and diffuse-scattered type gastric carcinomas. J Pathol 2002;198:55–59.
Young J, Biden KG, Simms LA, et al. HPP1: a transmembrane protein-encoding gene commonly methylated in colorectal polyps and cancers. Proc Natl Acad Sci USA 2001;98:265–270.
Shibata DM, Sato F, Mori Y, et al. Hypermethylation of HPP1 is associated with hMLH1 hypermethylation in gastric adenocarcinomas. Cancer Res 2002;62:5637–5640.
Byun DS, Lee MG, Chae KS, et al. Frequent epigenetic inactivation of RASSF1A by aberrant promoter hypermethylation in human gastric adenocarcinoma. Cancer Res 2001;61:7034–7038.
Ye M, Xia B, Guo Q, et al. Association of diminished expression of RASSF1A with promoter methylation in primary gastric cancer from patients of central China. BMC Cancer 2007;7:120.
Song SH, Jong HS, Choi HH, et al. Transcriptional silencing of Cyclooxygenase-2 by hyper-methylation of the 5′ CpG island in human gastric carcinoma cells. Cancer Res 2001;61:4628–4635.
Kikuchi T, Itoh F, Toyota M, et al. Aberrant methylation and histone deacetylation of cyclooxygenase 2 in gastric cancer. Int J Cancer 2002;97:272–277.
Satoh A, Toyota M, Itoh F, et al. Epigenetic inactivation of CHFR and sensitivity to microtubule inhibitors in gastric cancer. Cancer Res 2003;63:8606–8613.
Honda T, Tamura G, Waki T, et al. Promoter hypermethylation of the Chfr gene in neoplastic and non-neoplastic gastric epithelia. Br J Cancer 2004;90:2013–2016.
Takahashi T, Suzuki M, Shigematsu H, et al. Aberrant methylation of Reprimo in human malignancies. Int J Cancer 2005;115:503–510.
Bernal C, Aguayo F, Villarroel C, et al. Reprimo as a potential biomarker for early detection in gastric cancer. Clin Cancer Res 2008;14:6264–6269.
Jee CD, Kim MA, Jung EJ, et al. Identification of genes epigenetically silenced by CpG methylation in human gastric carcinoma. Eur J Cancer 2009;45:1282–1293.
Takada H, Wakabayashi N, Dohi O, et al. Tissue factor pathway inhibitor 2 (TFPI2) is frequently silenced by aberrant promoter hypermethylation in gastric cancer. Cancer Genet Cytogenet 2010;197:16–24.
Tokumaru Y, Nomoto S, Jeronimo C, et al. Biallelic inactivation of the RIZ1 gene in human gastric cancer. Oncogene 2003;22:6954–6958.
Oshimo Y, Oue N, Mitani Y, et al. Frequent epigenetic inactivation of RIZ1 by promoter hypermethylation in human gastric carcinoma. Int J Cancer 2004;110:212–218.
Miotto E, Sabbioni S, Veronese A, et al. Frequent aberrant methylation of the CDH4 gene promoter in human colorectal and gastric cancer. Cancer Res 2004;64:8156–8159.
Park J, Song SH, Kim TY, et al. Aberrant methylation of integrin alpha4 gene in human gastric cancer cells. Oncogene 2004;23:3474–3480.
Wei D, Gong W, Kanai M, et al. Drastic down-regulation of Kruppel-like factor 4 expression is critical in human gastric cancer development and progression. Cancer Res 2005;65:2746–2754.
Oshimo Y, Oue N, Mitani Y, et al. Frequent loss of RUNX3 expression by promoter hypermethylation in gastric carcinoma. Pathobiology 2004;71:137–143.
Gargano G, Calcara D, Corsale S, et al. Aberrant methylation within RUNX3 CpG island associated with the nuclear and mitochondrial microsatellite instability in sporadic gastric cancers. Results of a GOIM (Gruppo Oncologico dell’Italia Meridionale) prospective study. Ann Oncol 2007;suppl 6:vi103–vi109.
Kitajima Y, Ohtaka K, Mitsuno M, et al. Helicobacter pylori infection is an independent risk factor for Runx3 methylation in gastric cancer. Oncol Rep 2008;19:197–202.
Zou B, Chim CS, Zeng H, et al. Correlation between the singlesite CpG methylation and expression silencing of the XAF1 gene in human gastric and colon cancers. Gastroenterology 2006;131:1835–1843.
Tomii K, Tsukuda K, Toyooka S, et al. Aberrant promoter methylation of insulin-like growth factor binding protein-3 gene in human cancers. Int J Cancer 2007;120:566–573.
Ichikawa D, Koike H, Ikoma H, et al. Detection of aberrant methylation as a tumor marker in serum of patients with gastric cancer. Anticancer Res 2004;24:2477–2481.
Koike H, Ichikawa D, Ikoma H, et al. Comparison of methylationspecific polymerase chain reaction (MSP) with reverse transcriptase-polymerase chain reaction (RT-PCR) in peripheral blood of gastric cancer patients. J Surg Oncol 2004;87:182–186.
Leung WK, To KF, Chu ES, et al. Potential diagnostic and prognostic values of detecting promoter hypermethylation in the serum of patients with gastric cancer. Br J Cancer 2005;92:2190–2194.
Ikoma H, Ichikawa D, Koike H, et al. Correlation between serum DNA methylation and prognosis in gastric cancer patients. Anticancer Res 2006;26:2313–2316.
Ikoma H, Ichikawa D, Daito I, et al. Clinical application of methylation specific-polymerase chain reaction in serum of patients with gastric cancer. Hepatogastroenterology 2007;54:946–950.
Tan SH, Ida H, Lau QC, et al. Detection of promoter hypermethylation in serum samples of cancer patients by methylationspecific polymerase chain reaction for tumour suppressor genes including RUNX3. Oncol Rep 2007;18:1225–1230.
Abbaszadegan MR, Moaven O, Sima HR, et al. p16 promoter hypermethylation: a useful serum marker for early detection of gastric cancer. World J Gastroenterol 2008;14:2055–2060.
Wang YC, Yu ZH, Liu C, et al. Detection of RASSF1A promoter hypermethylation in serum from gastric and colorectal adenocarcinoma patients. World J Gastroenterol 2008;14:3074–3080.
Chen Z, Fan JQ, Li J, et al. Promoter hypermethylation correlates with the Hsulf-1 silencing in human breast and gastric cancer. Int J Cancer 2009;124:739–744.
Sakakura C, Hamada T, Miyagawa K, et al. Quantitative analysis of tumor-derived methylated RUNX3 sequences in the serum of gastric cancer patients. Anticancer Res 2009;29:2619–2625.
Koike H, Ichikawa D, Ikoma H, et al. Comparison of serum aberrant methylation and conventional tumor markers in gastric cancer patients. Hepatogastroenterology 2005;52:1293–1296.
Chen F, Kook H, Milewski R, et al. Hop is an unusual homeobox gene that modulates cardiac development. Cell 2002;110:713–723.
Shin CH, Liu ZP, Passier R, et al. Modulation of cardiac growth and development by HOP, an unusual homeodomain protein. Cell 2002;110:725–735.
Kook H, Lepore JJ, Gitler AD, et al. Cardiac hypertrophy and histone deacetylase-dependent transcriptional repression mediated by the atypical homeodomain protein Hop. J Clin Invest 2003;112:863–871.
Kee HJ, Kim JR, Nam KI, et al. Enhancer of polycomb1, a novel homeodomain only protein-binding partner, induces skeletal muscle differentiation. J Biol Chem 2007;282(10):7700–7709.
Asanoma K, Matsuda T, Kondo H, et al. NECC1, a candidate choriocarcinoma suppressor gene that encodes a homeodomain consensus motif. Genomics 2003;81:15–25.
Chen Y, Pacyna-Gengelbach M, Deutschmann N, et al. Homeobox gene HOP has a potential tumor suppressive activity in human lung cancer. Int J Cancer 2007;121:1021–1027.
Kendall J, Liu Q, Bakleh A, et al. Oncogenic cooperation and coamplification of developmental transcription factor genes in lung cancer. Proc Natl Acad Sci USA 2007;104:16663–16668.
Weir BA, Woo MS, Getz G, Perner S, Ding L, Beroukhim R, et al. Characterizing the cancer genome in lung adenocarcinoma. Nature 2007;450:893–898.
Kwei KA, Kim YH, Girard L, Kao J, Pacyna-Gengelbach M, Salari K, et al. Genomic profiling identifies TITF1 as a lineagespecific oncogene amplified in lung cancer. Oncogene 2008;27:3635–3640.
Ohki R, Nemoto J, Murasawa H, Oda E, Inazawa J, Tanaka N, et al. Reprimo, a new candidate mediator of the p53-mediated cell cycle arrest at the G2 phase. J Biol Chem 2000;275:22627–22630.
Hamilton JP, Sato F, Greenwald BD, Suntharalingam M, Krasna MJ, Edelman MJ, et al. Promoter methylation and response to chemotherapy and radiation in esophageal cancer. Clin Gastroenterol Hepatol 2006;4:701–708.
Tu W, Xu X, Peng L, et al. DAPK1 interaction with NMDA receptor NR2B subunits mediates brain damage in stroke. Cell 2010;140:222–234.
Inbal B, Cohen O, Polak-Charcon S, et al. DAP kinase links the control of apoptosis to metastasis. Nature 1997;390:180–184.
Jang CW, Chen CH, Chen CC, et al. TGF-beta induces apoptosis through Smad-mediated expression of DAP-kinase. Nature Cell Biol 2001;4:51–58.
Simpson DJ, Clayton RN, Farrell WE. Preferential loss of death associated protein kinase expression in invasive pituitary tumours is associated with either CpG island methylation or homozygous deletion. Oncogene 2002;21(8):1217–1224.
Tada Y, Wada M, Taguchi K, et al. The association of deathassociated protein kinase hypermethylation with early recurrence in superficial bladder cancers. Cancer Res 2002;62:4048–4053.
Sabbioni S, Miotto E, Veronese A, Sattin E, Gramantieri L, Bolondi L, et al. Multigene methylation analysis of gastrointestinal tumors: TPEF emerges as a frequent tumor-specific aberrantly methylated marker that can be detected in peripheral blood. Mol Diagn 2003;7:201–207.
Cheng YY, Yu J, Wong YP, Man EP, To KF, Jin VX, et al. Leung WK. Frequent epigenetic inactivation of secreted frizzled-related protein 2 (SFRP2) by promoter methylation in human gastric cancer. Br J Cancer 2007;97:895–901.
Jung Y, Park J, Bang YJ, Kim TY. Gene silencing of TSPYL5 mediated by aberrant promoter methylation in gastric cancers. Lab Invest 2008;88:153–160.
Kang GH, Lee S, Cho NY, Gandamihardja T, Long TI, Weisenberger DJ, et al. DNA methylation profiles of gastric carcinoma characterized by quantitative DNA methylation analysis. Lab Invest 2008;88:161–170.
Bernal C, Vargas M, Ossandon F, Santibanez E, Urrutia J, Luengo V, et al. DNA methylation profile in diffuse type gastric cancer: evidence for hypermethylation of the BRCA1 promoter region in early-onset gastric carcinogenesis. Biol Res 2008;41:303–315.
Kim M, Jang HR, Kim JH, Noh SM, Song KS, Cho JS, et al. Epigenetic inactivation of protein kinase D1 in gastric cancer and its role in gastric cancer cell migration and invasion. Carcinogenesis 2008;29:629–637.
Kim M, Lee KT, Jang HR, Kim JH, Noh SM, Song KS, et al. Epigenetic down-regulation and suppressive role of DCBLD2 in gastric cancer cell proliferation and invasion. Mol Cancer Res 2008;6:222–230.
Poplawski T, Tomaszewska K, Galicki M, Morawiec Z, Blasiak J. Promoter methylation of cancer-related genes in gastric carcinoma. Exp Oncol 2008;30:112–116.
Bernal C, Aguayo F, Villarroel C, Vargas M, Diaz I, Ossandon FJ, et al. Reprimo as a potential biomarker for early detection in gastric cancer. Clin Cancer Res 2008;14:6264–6269.
Cheng YY, Jin H, Liu X, Siu JM, Wong YP, Ng EK, et al. Fibulin1 is downregulated through promoter hypermethylation in gastric cancer. Br J Cancer 2008;99:2083–2087.
Buffart TE, Overmeer RM, Steenbergen RD, Tijssen M, van Grieken NC, Snijders PJ, et al. MAL promoter hypermethylation as a novel prognostic marker in gastric cancer. Br J Cancer 2008;99:1802–1807.
Wang LJ, Jin HC, Wang X, Lam EK, Zhang JB, Liu X, et al. ZIC1 is downregulated through promoter hypermethylation in gastric cancer. Biochem Biophys Res Commun 2009;379:959–963.
Tahara T, Arisawa T, Shibata T, Yamashita H, Yoshioka D, Hirata I. Effect of promoter methylation of multidrug resistance 1 (MDR1) gene in gastric carcinogenesis. Anticancer Res 2009;29:337–341.
Wu CS, Lu YJ, Li HP, Hsueh C, Lu CY, Leu YW, et al. Glutamate receptor, ionotropic, kainate 2 silencing by DNA hypermethylation possesses tumor suppressor function in gastric cancer. Int J Cancer 2009;126:2542–2552.
Wang X, Lau KK, So LK, Lam YW. CHD5 is down-regulated through promoter hypermethylation in gastric cancer. J Biomed Sci 2009;16:95.
Agarwal R, Mori Y, Cheng Y, Jin Z, Olaru AV, Hamilton JP, et al. Silencing of claudin-11 is associated with increased invasiveness of gastric cancer cells. PLoS One 2009;4:e8002.
Dohi O, Takada H, Wakabayashi N, Yasui K, Sakakura C, Mitsufuji S, et al. Epigenetic silencing of RELN in gastric cancer. Int J Oncol 2010;36:85–92.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yamashita, K., Sakuramoto, S. & Watanabe, M. Genomic and epigenetic profiles of gastric cancer: Potential diagnostic and therapeutic applications. Surg Today 41, 24–38 (2011). https://doi.org/10.1007/s00595-010-4370-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-010-4370-5