Abstract
Purpose
Cisplatin plus 5-fluorouracil has been globally accepted as a standard regimen for the treatment for advanced gastric cancer. However, cisplatin has several disadvantages, including renal toxicity and the need for admission. S-1 plus cisplatin has become a standard treatment for advanced gastric cancer in East Asia. This phase III study was designed to evaluate the potential benefits of adding docetaxel to S-1 without a platinum compound in patients with advanced gastric cancer.
Methods
Patients were randomly assigned to receive docetaxel plus S-1 or S-1 alone. The docetaxel plus S-1 group received docetaxel on day 1 and oral S-1 on days 1–14 of a 21-day cycle. The S-1 alone group received oral S-1 on days 1–28 of a 42-day cycle. The primary end point was overall survival.
Results
Of the 639 patients enrolled, 635 were eligible for analysis. The median overall survival was 12.5 months in the docetaxel plus S-1 group and 10.8 months in the S-1 alone group (p = 0.032). The median progression-free survival was 5.3 months in the docetaxel plus S-1 group and 4.2 months in the S-1 alone group (p = 0.001). As for adverse events, neutropenia was more frequent in the docetaxel plus S-1 group, but remained manageable.
Conclusion
As first-line treatment for advanced gastric cancer, docetaxel plus S-1 significantly improves median overall and progression-free survival as compared with S-1 alone. (ClinicalTrials.gov number: NCT00287768).
Similar content being viewed by others
Introduction
Gastric cancer is the second most common cause of cancer death worldwide. The only potentially curative treatment for patients with gastric cancer is surgical resection. However, regional and distant recurrence often occurs after surgery. The standard treatment for advanced or recurrent gastric cancer is chemotherapy, hoping to prolong survival.
In the late 1990s, cisplatin plus 5-fluorouracil was globally accepted as a benchmark treatment for advanced gastric cancer and has since been used in many controlled clinical trials of new chemotherapeutic regimens (Ohtsu et al. 2003; Van Cutsem et al. 2006; Al-Batran et al. 2008; Cunningham et al. 2008; Boku et al. 2009). However, cisplatin has several important drawbacks, including high incidences of nausea, vomiting (Kris et al. 2006), and renal toxicity (Fillastre and Raguenez-Viotte 1989; Arany and Safirstein 2003) the need for admission to receive therapy, and other adverse events negatively affecting the quality of life of patients. Cisplatin is contraindicated in patients with poor renal function. The development of combination chemotherapy regimens that do not include platinum compounds has thus been awaited as a new option for the first-line treatment for advanced gastric cancer.
During the planning phase of this trial, docetaxel (Mai et al. 1999; Graziano et al. 2000; Mavroudis et al. 2000; Bang et al. 2002) and S-1, an oral preparation combining tegafur (a prodrug of fluorouracil) with gimeracil and oteracil potassium in a molar ratio of 1:0.4:1, were shown to be effective as monotherapy against advanced gastric cancer (Sakata et al. 1998; Shirasaka 2009). In Western countries, a phase III study comparing docetaxel plus cisplatin/5-fluorouracil with cisplatin plus 5-fluorouracil was ongoing in patients with advanced gastric cancer (V325 study). In Japan, S-1 had become the most widely used drug for the treatment for advanced gastric cancer, and phase III studies of S-1 plus cisplatin versus S-1 alone (SPIRITS trial: Koizumi et al. 2008) and S-1 plus irinotecan versus S-1 alone (TOP-002 trial: Narahara et al. 2011) were ongoing.
Phase II and phase I/II studies of S-1 plus docetaxel in Japanese patients with advanced gastric cancer have reported response rates of 56.2 and 45.7 %, with median survival times of 14.3 and 14.0 months, respectively (Yoshida et al. 2006; Yamaguchi et al. 2006). Although the dose of docetaxel (40 mg per square meter of body surface area) was lower than that used in Western countries, treatment was well tolerated in both studies, suggesting that S-1 plus docetaxel is a promising new regimen for the chemotherapeutic management of advanced gastric cancer. To confirm and extend these results, we performed a controlled study comparing S-1 plus docetaxel with S-1 alone as first-line chemotherapy for advanced gastric cancer, without the use of any platinum compounds.
Patients and methods
The Japan Clinical Cancer Research Organization (JACCRO) GC-03 study (START trial) was a multicenter, prospective, randomized, phase III open-label trial performed by the JACCRO and the Korean Cancer Study Group (KCSG) ("Appendix"). Patients were registered and followed up using the FLADS® system (Takt Systems, Inc., Tokyo, Japan), a Web-based registration and data collection system for clinical trials of anticancer therapy.
This study was performed in accordance with the declaration of Helsinki and the ethical guidelines and regulations of each country. The protocol was approved by the ethics committees of each center before the initiation of enrollment. An independent Response and Safety Evaluation Committee reviewed all efficacy and safety data.
Written informed consent was obtained from all patients before enrollment in the study. Eligible patients had to satisfy the following criteria: an expected survival of 3 months or longer; no prior chemotherapy; an age of 20 to younger than 80 years; an Eastern Cooperative Oncology Group performance status of zero or one; a histologically confirmed diagnosis of unresectable or advanced gastric adenocarcinoma (including adenocarcinoma of the gastroesophageal junction) or unresectable recurrence; ability to orally intake food and liquids; either measurable or non-measurable lesions as defined by the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.0 (Therasse et al. 2000); and adequate organ functions.
Patients were excluded if they had any of the following conditions: another active cancer; severe ascites requiring drainage; grade 2 or severer peripheral neuropathy, pulmonary fibrosis, or interstitial pneumonitis; or a history of chemotherapy or radiotherapy for advanced gastric cancer.
Treatment and testing
Patients were stratified according to institutions and whether they had measurable or non-measurable lesions and were then randomly assigned to receive docetaxel plus S-1 or S-1 alone. Treatment was continued until the onset of progressive disease, the development of toxicity meeting the criteria for drug withdrawal, or the withdrawal of consent by the patient. The docetaxel plus S-1 group received docetaxel (40 mg per square meter of body surface area) as a 1-h intravenous infusion on day 1 and oral S-1 on days 1–14 of a 21-day cycle. The daily dose of S-1 (given in two divided doses) was assigned according to body surface area as follows: <1.25 m2, 80 mg daily; ≥1.25–<1.5 m2, 100 mg daily; and ≥1.5 m2, 120 mg daily. The S-1 alone group received the same dose levels of S-1 as the docetaxel plus S-1 group, similarly assigned according to body surface area, on days 1–28 of a 42-day cycle. In the event of predefined toxic events, protocol-specified treatment modifications were permitted. Adverse events were graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events (NCI-CTCAE), version 3.0.
Statistical analysis
The primary end point was overall survival, defined as the interval between enrollment and death from any cause. Secondary end points were progression-free survival, response rate, and safety. Response rates were based on the assessment of response by the investigators at each center and were reviewed by the Central Review Board of JACCRO; response was evaluated in accordance with RECIST, version 1.0.
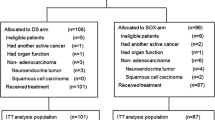
The overall survival and progression-free survival were calculated on the basis of an intent-to-treat analysis, and response rates were calculated on the basis of the per-protocol set (Fig. 1). Survival curves were calculated by the Kaplan–Meier method, and differences between groups were compared with the use of log-rank tests. In two previous phase II studies, the median survival times of patients with previously untreated advanced gastric cancer who received S-1 alone were 250 and 207 days (Sakata et al. 1998; Koizumi et al. 2000), respectively. Two phase II studies of docetaxel plus S-1 reported median survival times of 434 and 427 days, respectively (Yoshida et al. 2006; Yamaguchi et al. 2006). On the basis of these results, the median survival time was assumed to be 400 days in the docetaxel plus S-1 group and 300 days in the S-1 alone group. We estimated that a total enrollment of 628 patients was needed for the study to have a 90 % power to detect a difference in overall survival between the treatment groups with a two-sided alpha value of 0.05, assuming an enrollment period of 3 years, a follow-up period of 2 years, and a 10 % exclusion rate due to ineligibility. We planned an interim analysis after 377 deaths had been confirmed to decide whether to terminate the study.
This study was registered with ClinicalTrials.gov (ClinicalTrials.gov Identifier: NCT00287768).
Role of the funding source
JACCRO and KCSG employees contributed to the study design and data collection and interpretation. This study was supported by an unconditioned grant from Sanofi K.K. Japan. Sanofi K.K. Japan had no role in the study design, data collection, analysis, or interpretation, or in writing the manuscript or deciding whether it would be submitted for publication. Masahiro Takeuchi and Masashi Fujii had access to the raw data. The corresponding author had full access to all study data and final responsibility for the decision to submit for publication.
Results
Patients’ characteristics
Between September 2005 and September 2008, a total of 113 centers participated (97 in Japan and 16 in Korea), and a total of 639 patients were registered by means of the FLADS® system; 316 were assigned to the docetaxel plus S-1 group, and 323 were assigned to the S-1 alone group.
Four patients were ineligible because they did not have measurable or non-measurable lesions as defined by RECIST: two assigned to the docetaxel plus S-1 group and two assigned to the S-1 alone group. The intent-to-treat analysis thus included 635 patients, 314 in the docetaxel plus S-1 group and 321 in the S-1 alone group (Fig. 1). The baseline characteristics of the patients were similar in the two treatment groups (Table 1).
Efficacy
Median follow-up of intent-to-treat group (ITT) was 11.4 months (inter-quartile range 6.21–21.2). Of 635 cases, 582 died, 36 were still alive, and 17 were lost to follow-up. The median overall survival time was 12.5 months (95 % confidence interval (CI) 11.4–14.8) in the docetaxel plus S-1 group and 10.8 months (95 % CI 9.5–11.8) in the S-1 alone group. This difference in overall survival was statistically significant (p = 0.032; hazard ratio (HR) 0.84; 95 % CI 0.71–0.99) (Fig. 2a). Progression-free survival also differed significantly and was 5.3 months (95 % CI 4.5–5.9) in the docetaxel plus S-1 group and 4.2 months in the S-1 alone group (95 % CI 3.7–4.7; p < 0.001; HR 0.77; 95 % CI 0.65–0.90) (Fig. 2b). The response rate was calculated on the basis of the 480 (77.3 %) of the 621 patients in the per-protocol set. The response rate was 38.8 % (95 % CI 32.8–45.2; complete response, 3; partial response, 89) among the 237 patients with measurable lesions in the docetaxel plus S-1 group and 26.8 % (95 % CI 21.6–32.6; complete response, 5; partial response, 60) among the 243 patients with measurable lesions in the S-1 alone group. This difference was statistically significant (p = 0.005).
On subgroup analysis, docetaxel plus S-1 showed significantly better overall survival than S-1 alone in patients with a performance status of zero, patients with non-measurable lesions, patients with no lymph-node metastasis, and Japanese patients than in patients with performance status one, patients with measurable lesions, patients with lymph-node metastasis, and Korean patients (Figs. 3, 4). Peritoneal metastasis was found in 109 (76 %) of the 144 patients with non-measurable lesions.
Treatment and compliance
The median relative dose intensity was 80.4 % for docetaxel and 76.0 % for S-1 in the docetaxel plus S-1 group and 76.2 % for S-1 in the S-1 alone group. Treatment was delayed in 14.5 % of the patients in the docetaxel plus S-1 group and 4.2 % of those in the S-1 alone group. The main reason for treatment delays was adverse events in both groups. The main reason for withdrawal of treatment was disease progression in both groups. After withdrawal of the protocol treatment, second-line therapy was given to 69.7 % of the patients in the docetaxel plus S-1 group and 76.0 % of those in the S-1 group.
Among the Japanese patients who received second-line chemotherapy, 79 % were given irinotecan, cisplatin, or taxanes, while most of the 60 % of Korean patients who received second-line chemotherapy were given 5-fluorouracil-based regimens.
Safety
The incidence of grade 3 or higher adverse events was 58.1 % in the docetaxel plus S-1 group and 39.6 % in the S-1 alone group (p < 0.0001). The incidences of grade 3 or higher leukopenia and neutropenia were significantly higher in the docetaxel plus S-1 group than in the S-1 alone group (p < 0.0001) and that of febrile neutropenia was significantly higher in the docetaxel plus S-1 group than in the S-1 alone group (p = 0.0024; Table 2). There were two treatment-related deaths in the docetaxel plus S-1 group (0.6 %).
Discussion
Several pivotal phase III clinical trials have been performed in patients with advanced gastric cancer, including the V325 study of docetaxel plus cisplatin/5-fluorouracil by the V325 Study Group (Van Cutsem et al. 2006), a randomized trial of epirubicin plus cisplatin/5-fluorouracil by Cunningham and co-workers (Webb et al. 1997; Waters et al. 1999; Ross et al. 2002), and the SPIRITS trial of S-1 plus cisplatin by the SPRITS Trial Group (Koizumi et al. 2008). These trials have established the aforementioned regimens as standard treatments in their respective regions. All of these first-line regimens contain cisplatin. Our study in patients with advanced gastric cancer showed that adding docetaxel to S-1 significantly improved the overall survival, progression-free survival, and response rate as compared with S-1 alone. The survival benefit of docetaxel plus S-1 is particularly important, because regimens without platinum compounds would be a new option for the first-line treatment for advanced gastric cancer.
When we first reported the interim results of this trial at the American Society of Clinical Oncology (ASCO) Gastrointestinal Symposium 2012, the primary end point was not yet met. However, an independent statistician pointed out that data on more than 20 % of the patients had been censored and suggested that this problem should be solved to accurately evaluate treatment effectiveness. We therefore extended follow-up to 2 years after enrollment of the final patient, similar to the duration of follow-up at the initial analysis, to confirm the outcomes of the many patients with censored data. There was no alpha spending on reanalysis.
Docetaxel is a semisynthetic taxoid anticancer drug that is used as monotherapy or in combination with other anticancer agents to treat various cancers (Einzig et al. 1996; Chan et al. 1999; Shepherd et al. 2000; O’Shaughnessy et al. 2002; Fossella et al. 2003: Tannock et al. 2004; Martin et al. 2005; Marty et al. 2005; Posner et al. 2007). In the V325 study, docetaxel plus cisplatin/5-fluorouracil was compared with cisplatin/5-fluorouracil. The overall survival was significantly longer for docetaxel plus cisplatin/5-fluorouracil (9.2 months) than for cisplatin/5-fluorouracil (8.6 months; p = 0.02) (Van Cutsem et al. 2006). Although the doses of docetaxel differed between the V325 study (75 mg/m2) and our study (40 mg/m2), the benefit of adding docetaxel was confirmed in two large randomized studies of V325 and our study in advanced gastric cancer (Van Cutsem et al. 2006).
The main toxic effect of docetaxel is myelosuppression. In the V325 study, the incidence of severe neutropenia was 82 % in patients who received docetaxel plus cisplatin/5-fluorouracil, approved in Western countries for the treatment for advanced gastric cancer (Van Cutsem et al. 2006). Many modified regimens of docetaxel plus cisplatin/5-fluorouracil with better safety profiles can be used (Roth et al. 2007; Lorenzen et al. 2007; Tebbutt et al. 2010; Shah et al. 2010; Inal et al. 2012). In our study, we used docetaxel in a dose of 40 mg/m2, which was based on the results of Japanese phase I/II studies, and grade 3 or higher neutropenia occurred in only 29 % of the patients who received docetaxel plus S-1. Because the rate of severe neutropenia was low in our study, the dose of docetaxel might be able to be increased slightly, approaching the level used in Western countries, but since docetaxel plus S-1 therapy was administered on an outpatient basis, our dose setting might be appropriate for Asian patients and is considered effective in terms of survival.
The tumor burden is a more important prognostic factor than other factors, such as performance status or whether the patient receives adjuvant chemotherapy. To our knowledge, the present study is the first large randomized clinical trial to stratify patients with advanced gastric cancer according to measurable or non-measurable lesions. In our pre-planned subgroup analysis, there was no statistically significant difference in overall survival between the docetaxel plus S-1 group (11.7 months; n = 242) and the S-1 alone group (10.3 months; n = 249; HR 0.90; p = 0.28) among patients with measurable lesions. Among patients with non-measurable lesions, however, the overall survival was significantly longer in the docetaxel plus S-1 group (17.9 months; n = 72) than in the S-1 alone group (12.0 months; n = 72; HR 0.65; p = 0.013) (Fig. 3). In an exploratory subgroup analysis of the SPIRITS trial, S-1 plus cisplatin was associated with a higher benefit in patients with non-target tumors than in those with target tumors (Koizumi et al. 2008).
For pre-stratified measurable tumors, the overall survival benefit of docetaxel plus S-1 was not significant, but progression-free survival was significantly better with docetaxel plus S-1 (4.7 months) than with S-1 alone (3.9 months; HR 0.82; p = 0.03). This finding might be attributed to the notion that overall survival time is influenced by effective second-line chemotherapy, whereas progression-free survival is not. For patients with measurable tumors, more active regimens for combination chemotherapy, such as triplet regimens or regimens including new biological agents, should be investigated.
The present study was conducted in Japan and Korea, and the recommended dose of S-1 differs between Asian patients (80 mg/m2) and those in Western countries (50 mg/m2) (Ajani et al. 2010). Our results therefore cannot be extrapolated to all patients with gastric cancer. However, docetaxel plus S-1 therapy appears to be a promising, non-platinum-based treatment option for patients with gastric cancer in East Asia, which accounts for about 60 % of all cases of gastric cancer in the world. The results of our study may also provide important clues to the development of S-1-based, non-platinum regimens in Europe and North America.
In conclusion, docetaxel plus S-1 therapy is expected to become an important treatment option for patients with advanced gastric cancer, particularly those with compromised renal function or who want to receive chemotherapy on an outpatient basis.
References
Ajani JA, Rodriguez W, Bodoky G, Moiseyenko V, Lichinitser M, Gorbunova V, Vynnychenko I, Garin A, Lang I, Falcon S (2010) Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol 28:1547–1553
Al-Batran SE, Hartmann JT, Probst S et al (2008) Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol 26:1435–1442
Arany I, Safirstein RL (2003) Cisplatin nephrotoxicity. Semin Nephrol 23:460–464
Bang YJ, Kang WK, Kang YK et al (2002) Docetaxel 75 mg/m2 is active and well tolerated in patients with metastatic or recurrent gastric cancer: a phase II trial. Jpn J Clin Oncol 32:248–254
Boku N, Yamamoto S, Fukuda H et al (2009) Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomized phase 3 study. Lancet Oncol 10:1063–1069
Chan S, Friedrichs K, Noel D et al (1999) Prospective randomized trial of docetaxel versus doxorubicin in patients with metastatic breast cancer. J Clin Oncol 17:2341–2354
Cunningham D, Starling N, Rao S et al (2008) Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 358:36–46
Einzig AI, Neuberg D, Remick SC et al (1996) Phase II trial of docetaxel (Taxotere®) in patients with adenocarcinoma of the upper gastrointestinal tract previously untreated with cytotoxic chemotherapy: the Eastern Cooperative Oncology Group (ECOG). Med Oncol 13:87–93
Fillastre JP, Raguenez-Viotte G (1989) Cisplatin nephrotoxicity. Toxicol Lett 46:163–175
Fossella F, Pereira JR, von Pawel J et al (2003) Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the TAX 326 study group. J Clin Oncol 21:3016–3024
Graziano F, Catalano V, Baldelli AM et al (2000) A phase II study of weekly docetaxel as salvage chemotherapy for advanced gastric cancer. Ann Oncol 11:1263–1266
Inal A, Kaplan MA, Kucukoner M, Isikdogan A (2012) Docetaxel and cisplatin plus fluorouracil compared with modified docetaxel, cisplatin, and 5-fluorouracil as first-line therapy for advanced gastric cancer: a retrospective analysis of single institution. Neoplasma 59:233–236
Koizumi W, Kurihara M, Nakano S, Hasegawa K (2000) Phase II study of S-1, a novel oral derivative of 5-fluorouracil, in advanced gastric cancer. For the S-1 Cooperative Gastric Cancer Study Group. Oncology 58:191–197
Koizumi W, Narahara H, Hara T et al (2008) S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 9:215–221
Kris MG, Hesketh PJ, Somerfield MR et al (2006) American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol 24:2932–2947
Lorenzen S, Hentrich M, Haberl C et al (2007) Split-dose docetaxel, cisplatin and leucovorin/fluorouracil as first-line therapy in advanced gastric cancer and adenocarcinoma of the gastroesophageal junction: results of a phase II trial. Ann Oncol 18:1673–1679
Mai M, Sakata Y, Kanamaru R et al (1999) A late phase II clinical study of RP56976 (docetaxel) in patients with advanced or recurrent gastric cancer: a Cooperative Study Group trial (group B). Jpn J Cancer Chemother 26:487–496 [Japanese]
Martin M, Pienkowski T, Mackey J et al (2005) Adjuvant docetaxel for node-positive breast cancer. N Engl J Med 352:2302–2313
Marty M, Cognetti F, Maraninchi D et al (2005) Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol 23:4265–4274
Mavroudis D, Kourousis C, Androulakis N et al (2000) Frontline treatment of advanced gastric cancer with docetaxel and granulocyte colony-stimulating factor (G-CSF): a phase II trial. Am J Clin Oncol 23:341–344
Narahara H, Iishi H, Imamura H et al (2011) Randomized phase III study comparing the efficacy and safety of irinotecan plus S-1 with S-1 alone as first-line treatment for advanced gastric cancer (study GC0301/TOP-002). Gastric Cancer 14:72–80
Ohtsu A, Shimada Y, Shirao K, et al (2003) Randomized phase III trial of fluorouracil alone versus fluorouracil plus cisplatin versus uracil and tegafur plus mitomycin in patients with unresectable, advanced gastric cancer: the Japan clinical oncology group study (JCOG9205). J Clin Oncol 21:54–59
O’Shaughnessy J, Miles D, Vukelja S et al (2002) Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: phase III trial results. J Clin Oncol 20:2812–2823
Posner MR, Hershock DM, Blajman CR et al (2007) Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med 357:1705–1715
Ross P, Nicolson M, Cunningham D et al (2002) Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) with epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol 20:1996–2004
Roth AD, Fazio N, Stupp R et al (2007) Docetaxel, cisplatin, and fluorouracil; docetaxel and cisplatin; and epirubicin, cisplatin, and fluorouracil as systemic treatment for advanced gastric carcinoma: a randomized phase II trial of the Swiss Group for Clinical Cancer Research. J Clin Oncol 25:3217–3223
Sakata Y, Ohtsu A, Horikoshi N, Sugimachi K, Mitachi Y, Taguchi T (1998) Late phase II study of novel oral fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1 M otastat potassium) in advanced gastric cancer patients. Eur J Cancer 34:1715–1720
Shah MA, Shibata S, Stoller RG et al (2010) Random assignment multicenter phase II study of modified docetaxel, cisplatin, fluorouracil (mDCF) versus DCF with growth factor support (GCSF) in metastatic gastroesophageal adenocarcinoma (GE). J Clin Oncol 28(Suppl):15s abstract
Shepherd FA, Dancey J, Ramlau R et al (2000) Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 18:2095–2103
Shirasaka T (2009) Development history and concept of an oral anticancer agent S-1 (TS-1): its clinical usefulness and future vistas. Jpn J Clin Oncol 39:2–15
Tannock IF, de Wit R, Berry WR et al (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351:1502–1512
Tebbutt NC, Cummins MM, Sourjina T et al (2010) Randomised, non-comparative phase II study of weekly docetaxel with cisplatin and 5-fluorouracil or with capecitabine in oesophagogastric cancer: the AGITG ATTAX trial. Br J Cancer 102:475–481
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Van Cutsem E, Moiseyenko VM, Tjulandin S et al (2006) Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 Study group. J Clin Oncol 24:1991–4997
Waters JS, Norman A, Cunningham D et al (1999) Long-term survival after epirubicin, cisplatin and fluorouracil for gastric cancer: results of a randomized trial. Br J Cancer 80:269–272
Webb A, Cunningham D, Scarffe JH et al (1997) Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol 15:261–267
Yamaguchi K, Shimamura T, Hyodo I et al (2006) Phase I/II study of docetaxel and S-1 in patients with advanced gastric cancer. Br J Cancer 94:1803–1808
Yoshida K, Ninomiya M, Takakura N et al (2006) Phase II study of docetaxel and S-1 combination therapy for advanced or recurrent gastric cancer. Clin Cancer Res 12:3402–3407
Acknowledgments
This study was supported by the Japan Clinical Cancer Research Organization (JACCRO) and Korean Cancer Study Group (KCSG). We thank all of our patients and their families, as well as all of the site investigators. We are grateful to Dr. Y. Shimada of National Cancer Center Hospital and to Dr. T. Sasaki of Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital for their kind advice as members of the Independent Data Monitoring Committee. The authors are indebted to Prof. J. Patrick Barron of the Department of International Medical Communications of Tokyo Medical University for his review of this manuscript.
Conflict of interest
Yeul Hong Kim has received the grants from Sanofi, Jeil Pharmaceutical, Pfizer, Astra Zeneca, Merck Serono, Novartis, and Roche, as well as honoraria for lectures from Sanofi, Jeil Pharmaceutical, Merck Serono, and Roche and a travel grant from Sanofi. Masashi Fujii has received consulting fees and honoraria for lectures from Taiho Pharmaceutical. Hyun Cheol Chung has received a grant from Sanofi. Akinori Takagane has received travel and hotel grants from JACCRO. Kazuhiro Yoshida has received grants from Chugai Pharmaceutical, Kyowa Hakko Kirin, Pfizer, Sanofi, Taiho Pharmaceutical, and Yakult Honsha, as well as honoraria for lectures from Chugai Pharmaceutical, Kyowa Hakko Kirin, Novartis, Pfizer, Sanofi, Taiho Pharmaceutical, and Yakult Honsha. He also has consultant or advisory relationships with Taiho Pharmaceutical and Roche. All other authors have declared no conflicts of interest.
Author information
Authors and Affiliations
Consortia
Corresponding author
Appendix
Appendix
Chief investigator: M. Fujii.
Members of the JACCRO and KCSG study teams were as follows:
The protocol design group: H. Baba, H. Takiuchi, K. Yoshida, T. Kubota, W. Koizumi; the efficacy and safety assessment committee: M. Takeuchi, T. Sasaki, Y. Shimada; statistical analyst: M. Takeuchi; principal investigators: (JACCRO group in Japan) Aichi Cancer Center—K. Muro, T. Ura; Aichi Medical University—K. Suzumura; Akita University Hospital—M. Odajima; Aomori Prefectural Central Hospital—S. Saitoh; Asahikawa Kosei Hospital—M. Goto; Asahikawa Medical University Hospital—K. Moriichi, T. Hoshi; Cancer Institute Hospital—K. Chin, M. Suenaga, Y. Kuboki; Dokkyo Medical University—K. Miyachi; Ehime University Hospital—Y. Kojima; Fukui Prefectural Hospital—O. Hosokawa; Fukui Red Cross Hospital—H. Fujii; Fukushima Medical University—Z. Sase; Fukuyama City Hospital—S. Asami; Gifu Municipal Hospital—H. Oshita; Gifu Prefectural General Medical Center—H. Kato; Gifu University School of Medicine—F. Sakashita, K. Yamaguchi; Gunma Prefectural Cancer Center—H. Hosaka, N. Haga; Gunma University Graduate School of Medicine—R. Aihara; Hakodate Goryokaku Hospital—A. Takagane; Hirosaki University Graduate School of Medicine—H. Kawasaki, J. Itoh, T. Takahata; Hiroshima City Asa Hospital—N. Hirabayashi; Hiroshima Red Cross Hospital and Atomic-bomb Survivors Hospital—M. Yamamoto; Hiroshima University—K. Tanabe; Hospital, University of the Ryukyus—M. Nakamoto; Hyogo College of Medicine—Y. Fujiwara; Ibi Kosei Hospital—S. Tachibana; Iizuka Hospital—T. Nagaie; International University of Health and Welfare Mita Hospital—K. Ohta; Iwate Medical University—K. Koeda; Jichi Medical University Hospital—M. Hyodo, Y. Hosoya; Jikei University School of Medicine—T. Sasaki; Kagoshima University Graduate School of Medical and Dental Sciences—A. Nakajo; Kanazawa Medical University—T. Kosaka; Kanazawa University—T. Fujimura; Kaneda Hospital—M. Uno; Keiju Medical Center—T. Kamata; Kinki Central Hospital—K. Kobayashi; Kinki University School of Medicine—M. Miyazaki, T. Sato, W. Okamoto; Kitasato University East Hospital—K. Higuchi, S. Tanabe, T. Sasaki, W. Koizumi; Kobe University Hospital—Y. Fujishima; Kochi Health Sciences Center—A. Tsuji; Kouseiren Takaoka Hospital—T. Hara; Kumamoto University Graduate School of Medical Sciences—M. Watanabe; Kurume University, School of Medicine—A. Keishiro; Kushiro City General Hospital—A. Goto; Kyorin University Hospital—O. Yanagida; Kyoto University Hospital—S. Matsumoto; Kyushu Central Hospital—K. Sumiyoshi; Kyusyu University Graduate School of Medicine—H. Saeki; Matsuyama Shimin Hospital—R. Watanabe, S. Yunoki; Minoh City Hospital—S. Iijima; Morioka Red Cross Hospital—Y. Sugimura; Nagano Municipal Hospital—K. Okita, Y. Munakata; Nagasaki University Graduate School of Biomedical Science—S. Hidaka; Nakadori General Hospital—H. Andoh; National Center for Global Health and Medicine—J. Akiyama; National Hospital Organization Hokkaido Cancer Center—Y. Takahashi; National Hospital Organization Kure Medical Center and Chugoku Cancer Center—N. Hatanaka; National Hospital Organization Nagasaki Medical Center—T. Nakata; National Hospital Organization Nagoya Medical Center—M. Kataoka; National Kyushu Cancer Center—H. Ariyama, T. Esaki; Nihon University School of Medicine—M. Kochi; Nippon Steel Yawata Memorial Hospital—R. Ohta; Osaka City University Graduate School of Medicine—B. Nakata; Osaka General Medical Center—K. Nishikawa; Rinku General Medical Center, Izumisano Municipal Hospital—H. Mizuno, M. Izukura, T. Mizushima; Saga University—M. Tanaka; Saiseikai Shiga Prefectural Hospital—T. Shigematsu; SaiseikaiYokohamashi Tobu Hospital—T. Egawa; Sakai Municipal Hospital—T. Kishimoto; Sapporo Medical University School of Medicine—T. Nobuoka; Shimane University School of Medicine—N. Hirahara; Showa University Fujigaoka Hospital—H. Nemoto; Showa University Hospital—K. Yamazaki, Y. Tajima; Social Insurance Chuo General Hospital, M. Shibasaki; St.Marianna University School of Medicine—T. Tsuda; St.Marianna University Yokohama-city Seibu Hospital—A. Hanai, K. Sumiyoshi, M. Kimura; Surugadai Nihon University Hospital—K. Hagiwara; Teikyo University School of Medicine—K. Iwasaki; Tohoku Rosai Hospital—H. Musha; Tohoku University—Y. Kakudo; Tokai University Hospital—K. Nabeshima; Toki Municipal General Hospital—T. Oiwa; Tokushima Red Cross Hospital—H. Ishikura, H. Okitsu; Tokyo Medical and Dental University—M. Inokuchi, S. Ooka; Tokyo Medical University—S. Hoshino; Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital—Y. Iwasaki; Toranomon Hospital—T. Iizuka; Tottori University Faculty of Medicine—H. Saito, S. Tatebe; Toyonaka Municipal Hospital—J. Fujiita; University of Occupational and Environmental Health—A. Higure, K. Hirata; Yamagata Prefectural Central Hospital—H. Saito, K. Suzuki, T. Matsuda; Yamanashi Prefectural Central Hospital—M. Hada; Yokohama Municipal Citizen’s Hospital—M. Takahashi; (KCSG group in South Korea) Chungnam National University Hospital—S. Y. Kim; Department of Internal Medicine St. Mary’s Hospital—I. S. Woo; Hallym University Sacred Heart Hospital—D. Y. Zang; Hanyang University Guri Hospital—J. H. Choi; Inha University—M. H. Lee; Inje University Seoul Paik Hospital—H. G. Lee; Kangbuk Samsung Hospital—S. S. Lee; Korea University Anam Hospital—Y. H. Kim; Korea University Guro Hospital—J. S. Kim; Seoul National University Bundang Hospital—G. W. Lee; Seoul National University Hospital—T. Y. Kim; St. Vincent’s Hospital—H. K. Kim; Wonju Christian Hospital—J. I. Lee, K. Y. Shim; Yeungnam University Hospital—K. H. Lee; Yong Dong Severance Hospital—H. C. Jeung; Yonsei University College of Medicine—S. J. Kim.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Koizumi, W., Kim, Y.H., Fujii, M. et al. Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: a randomized study (START). J Cancer Res Clin Oncol 140, 319–328 (2014). https://doi.org/10.1007/s00432-013-1563-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-013-1563-5