Abstract
Purpose
The aim of this study is to confirm the predictive value of controlling nutritional status (CONUT), as a postoperative prognostic marker for esophageal cancer patients undergoing esophagectomy.
Methods
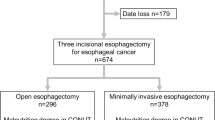
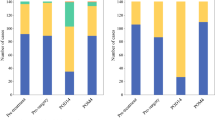
We retrospectively analyzed 373 patients who underwent three-incision esophagectomy with 2- or 3-field lymphadenectomy for esophageal cancer between April 2005 and March 2016. The patients were divided into three groups based on the degree of preoperative malnutrition as assessed by CONUT: normal, light malnutrition, and moderate or severe malnutrition.
Results
The patients with moderate or severe malnutrition experienced a significantly higher frequency of reoperation (normal or light malnutrition, 6.3%; moderate or severe malnutrition, 18.2%; P = 0.033) and a higher tendency for respiratory morbidities (normal or light malnutrition, 14.0%; moderate or severe malnutrition, 27.3%; P = 0.088). Cox regression analysis identified a significantly poor prognosis, in both overall survival (hazard ratio (HR), 3.56; 95% confidence interval (CI), 1.714–7.390; P < 0.001) and cancer-specific survival (HR, 3.41; 95% CI, 1.790–6.516; P = 0.046).
Conclusions
CONUT is convenient and useful for preoperatively assessing malnutrition and prognosis of esophageal cancer patients who underwent surgery.


Similar content being viewed by others
References
Vashist YK, Loos J, Dedow J et al (2011) Glasgow prognostic score is a predictor of perioperative and long-term outcome in patients with only surgically treated esophageal cancer. Ann Surg Oncol 18:1130–1138
Kosumi K, Baba Y, Ishimoto T et al (2016) Neutrophil/lymphocyte ratio predicts the prognosis in esophageal squamous cell carcinoma patients. Surg Today 46:405–413
Feng JF, Huang Y, Chen QX (2014) Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinoma. World J Surg Oncol 12:58
Huang Y, Feng JF (2015) Low preoperative lymphocyte to monocyte ratio predicts poor cancer-specific survival in patients with esophageal squamous cell carcinoma. Onco Targets Ther 8:137–145
Harada K, Ida S, Baba Y, Ishimoto T et al (2015) Prognostic and clinical impact of sarcopenia in esophageal squamous cell carcinoma. Dis Esophagus. doi:10.1111/dote.12381
Watanabe M, Ishimoto T, Baba Y, Nagai Y, Yoshida N, Yamanaka T, Baba H (2013) Prognostic impact of body mass index in patients with squamous cell carcinoma of the esophagus. Ann Surg Oncol 20:3984–3991
Scarpa M, Filip B, Cavallin F, Alfieri R, Saadeh L, Cagol M, Castoro C (2015) Esophagectomy in elderly patients: which is the best prognostic score? Dis Esophagus. doi:10.1111/dote.12358
Ignacio de Ulíbarri J, González-Madroño A, de Villar NG et al (2005) CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp 20:38–45
Yoshida N, Baba Y, Shigaki H et al (2016) Preoperative nutritional assessment by controlling nutritional status (CONUT) is useful to estimate postoperative morbidity after esophagectomy for esophageal cancer. World J Surg 40:1910–1917
Edge S, Byrd DR, Compton CC, Fritz AG, Green FL, Trotti A (2009) AJCC cancer staging manual, seventh edn. Springer, New York
Society of Thoracic Surgeons (2013) Risk-adjusted morbidity and mortality for esophagectomy for cancer. Available: http://www.sts.org/quality-research-patient-safety/quality/quality-performance-measures Accessed 24 Aug 2016
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Wu N, Chen G, Hu H, Pang L, Chen Z (2015) Low pretherapeutic serum albumin as a risk factor for poor outcome in esophageal squamous cell carcinomas. Nutr Cancer 67:481–485
de Martino M, Leitner CV, Seemann C et al (2015) Preoperative serum cholesterol is an independent prognostic factor for patients with renal cell carcinoma (RCC). BJU Int 115:397–404
Tomita M, Ayabe T, Shimizu T, Nakamura K (2012) Preoperative total serum cholesterol and patients’ survival in resected nonsmall cell lung cancer. Lung Cancer Int. doi:10.1155/2012/463520
Kotani K, Sekine Y, Ishikawa S, Ikpot IZ, Suzuki K, Remaley AT (2013) High-density lipoprotein and prostate cancer: an overview. J Epidemiol 23:313–319
Kuroda K, Nakashima J, Kanao K et al (2007) Interleukin 6 is associated with cachexia in patients with prostate cancer. Urology 69:113–117
Kumari N, Dwarakanath BS, Das A, Bhatt AN (2016) Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. doi:10.1007/s13277–016–5098–7
Feng JF, Liu JS, Huang Y (2014) Lymphopenia predicts poor prognosis in patients with esophageal squamous cell carcinoma. Medicine (Baltimore) 93:e257
Balmanoukian A, Ye X, Herman J, Laheru D, Grossman SA (2012) The association between treatment-related lymphopenia and survival in newly diagnosed patients with resected adenocarcinoma of the pancreas. Cancer Investig 30:571–576
Campian JL, Sarai G, Ye X, Marur S, Grossman SA (2014) Association between severe treatment-related lymphopenia and progression-free survival in patients with newly diagnosed squamous cell head and neck cancer. Head Neck 36:1747–1753
Aggarwal S, Gollapudi S, Gupta SJ (1999) Increased TNF-alpha-induced apoptosis in lymphocytes from aged humans: changes in TNF-alpha receptor expression and activation of caspases. Immunol 162:2154–2161
Kou F, Lu Z, Li J et al (2016) Pretreatment lymphopenia is an easily detectable predictive and prognostic marker in patients with metastatic esophagus squamous cell carcinoma receiving first-line chemotherapy. Cancer Med 5:778–586
Ray-Coquard I, Cropet C, Van Glabbeke M et al (2009) Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res 69:5383–5391
Cézé N, Thibault G, Goujon G, Viguier J, Watier H, Dorval E, Lecomte T (2011) Pre-treatment lymphopenia as a prognostic biomarker in colorectal cancer patients receiving chemotherapy. Cancer Chemother Pharmacol 68:1305–1313
Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S, Piantadosi S, NABTT CNS Consortium (2011) Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res 17:5473–5480
He JR, Shen GP, Ren ZF et al (2012) Pretreatment levels of peripheral neutrophils and lymphocytes as independent prognostic factors in patients with nasopharyngeal carcinoma. Head Neck 34:1769–1776
Kobayashi N, Usui S, Kikuchi S, Goto Y, Sakai M, Onizuka M, Sato Y (2012) Preoperative lymphocyte count is an independent prognostic factor in node-negative non-small cell lung cancer. Lung Cancer 75:223–227
Fuhrman MP (2002) The albumin-nutrition connection: separating myth from fact. Nutrition 18:199–200
Kondrup J, Rasmussen HH, Hamberg O, Stanga Z, Ad Hoc ESPEN Working Group (2003) Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr 22:321–336
Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, Jeejeebhoy KN (1987) What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr 11:8–13
Kataoka K, Takeuchi H, Mizusawa J et al (2016) Prognostic impact of postoperative morbidity after esophagectomy for esophageal cancer: exploratory analysis of JCOG9907. Ann Surg. doi:10.1097/SLA.0000000000001828
Yamashita K, Makino T, Miyata H et al (2016) Postoperative infectious complications are associated with adverse oncologic outcomes in esophageal cancer patients undergoing preoperative chemotherapy. Ann Surg Oncol 23:2106–2114
Baba Y, Yoshida N, Shigaki H et al (2016) Prognostic impact of postoperative complications in 502 patients with surgically resected esophageal squamous cell carcinoma: a retrospective single institution study. Ann Surg 264:305–311
Markar S, Gronnier C, Duhamel A et al (2015) The impact of severe anastomotic leak on long-term survival and cancer recurrence after surgical resection for esophageal malignancy. Ann Surg 262:972–980
van der Schaaf M, Derogar M, Johar A et al (2014) Reoperation after oesophageal cancer surgery in relation to long-term survival: a population-based cohort study. BMJ Open 4:e004648
Fukuda T, Seto Y, Yamada K, Hiki N, Fukunaga T, Oyama S, Yamaguchi T (2008) Can immune-enhancing nutrients reduce postoperative complications in patients undergoing esophageal surgery? Dis Esophagus 21:708–711
Mazaki T, Ishii Y, Murai I (2015) Immunoenhancing enteral and parenteral nutrition for gastrointestinal surgery: a multiple-treatments meta-analysis. Ann Surg 261:662–669
Kojima S, Sakakibara H, Motani S et al (2007) Incidence of chronic obstructive pulmonary disease, and the relationship between age and smoking in a Japanese population. J Epidemiol 17:54–60
Yoshida N, Watanabe M, Baba Y et al (2014) Risk factors for pulmonary complications after esophagectomy for esophageal cancer. Surg Today 44:526–532
Acknowledgements
No funding was received for this study.
Authors’ contributions
Study conception and design: Naoya Yoshida, Kazuto Harada, and Hideo Baba. Acquisition of data: Naoya Yoshida, Yoshifumi Baba, Masaaki Iwatsuki, Keisuke Kosumi, Koichi Kinoshita, Kenichi Nakamura, Yasuo Sakamoto, Yuji Miyamoto, Ryuichi Karashima, Kosuke Mima, Hiroshi Sawayama, Mayuko Ohuchi, and Akira Chikamoto. Analysis and interpretation of data: Naoya Yoshida, Kazuto Harada, and Yu Imamura. Drafting of manuscript: Naoya Yoshida, Masayuki Watanabe, and Hideo Baba. Critical revision of manuscript: Hideo Baba.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Yoshida, N., Harada, K., Baba, Y. et al. Preoperative controlling nutritional status (CONUT) is useful to estimate the prognosis after esophagectomy for esophageal cancer. Langenbecks Arch Surg 402, 333–341 (2017). https://doi.org/10.1007/s00423-017-1553-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-017-1553-1