Abstract
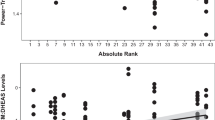
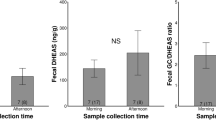
Stress and animal well-being are often assessed using concentrations of glucocorticoids (GCs), a product of the hypothalamic–pituitary–adrenal axis. However, GC concentrations can also be modulated by predictable events, such as changes in season or life history stage. Understanding normative patterns of adrenal activity is critical for making valid conclusions about changes in GC concentrations. In this study, we validated an assay for monitoring fecal glucocorticoid metabolites (FGM) in Canada lynx. We then used this technique to assess patterns of adrenal activity in Canada lynx across several contexts. Our results show that captive lynx have higher FGM concentrations than wild lynx, which may be related to differences in stress levels, metabolic rate, diet, or body condition. We also found that FGM concentrations are correlated with reproductive status in females, but not in males. For males, seasonal increases in FGM expression coincide with the onset of the breeding season, whereas in females, FGM increase toward the end of the breeding season. This information provides a valuable foundation for making inferences about normative versus stress-induced changes in adrenal activity in Canada lynx.




Similar content being viewed by others
References
Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP (2009) The pathogenetic role of cortisol in the metabolic syndrome: a hypothesis. J Clin Endocrinol Metab 94:2692–2701
Atkinson HC, Waddell BJ (1997) Circadian variation in basal plasma corticosterone and adrenocorticotropin in the rat: sexual dimorphism and changes across the estrous cycle. Endocrinology 138:3842–3848
Boonstra R (2004) Coping with changing northern environments: the role of the stress axis in birds and mammals. Integr Comp Biol 44:95–108
Brann D, Mahesh V (1991) Role of corticosteroids in female reproduction. FASEB J 5:2691–2698
Cavigelli SA (1999) Behavioural patterns associated with faecal cortisol levels in free-ranging female ring-tailed femurs, Lemur catta. Anim Behav 57:935–944
Cavigelli SA, Monfort SL, Whitney TK, Mechref YS, Novotny M, McClintock MK (2005) Frequent serial fecal corticoid measures from rats reflect circadian and ovarian corticosterone rhythms. J Endocrinol 184:153–163
Chrousos GP, Torpy DJ, Gold PW (1998) Interactions between the hypothalamic–pituitary–adrenal axis and the female reproductive system: clinical implications. Ann Intern Med 129:229–240
Cyr NE, Romero LM (2008) Fecal glucocorticoid metabolites of experimentally stressed captive and free-living starlings: implications for conservation research. Gen Comp Endocrinol 158:20–28
Dantzer B, McAdam AG, Palme R, Fletcher QE, Boutin S, Humphries MM, Boonstra R (2010) Fecal cortisol metabolite levels in free-ranging North American red squirrels: assay validation and the effects of reproductive condition. Gen Comp Endocrinol 167:279–286
Dehnhard M, Fanson K, Frank A, Naidenko SV, Vargas A, Jewgenow K (2010) Comparative metabolism of gestagens and estrogens in the four lynx species, the Eurasian (Lynx lynx), the Iberian (L. pardinus), the Canada lynx (L. canadensis) and the bobcat (L. rufus). Gen Comp Endocrinol 167:287–296
Fanson KV (2009) Stress and reproductive physiology in Canada lynx (Lynx canadensis): implications for in situ and ex-situ conservation. PhD Dissertation, Purdue University, West Lafayette, Indiana
Fanson KV, Wielebnowski NC, Shenk TM, Jakubas WJ, Squires JR, Lucas JR (2010a) Patterns of testicular activity in captive and wild Canada lynx (Lynx canadensis). Gen Comp Endocrinol 169:210–216
Fanson KV, Wielebnowski NC, Shenk TM, Vashon JH, Squires JR, Lucas JR (2010b) Patterns of ovarian and luteal activity in captive and wild Canada lynx (Lynx canadensis). Gen Comp Endocrinol 169:217–224
Frigerio D, Dittami J, Möstl E, Kotrschal K (2004) Excreted corticosterone metabolites co-vary with ambient temperature and air pressure in male Greylag geese (Anser anser). Gen Comp Endocrinol 137:29–36
Goymann W (2005) Noninvasive monitoring of hormones in bird droppings—physiological validation, sampling, extraction, sex differences, and the influence of diet on hormone metabolite levels. Ann N Y Acad Sci 1046:35–53
Hajamor S, Despres JP, Couillard C, Lemieux S, Tremblay A, Prud’homme D, Tchernof A (2003) Relationship between sex hormone-binding globulin levels and features of the metabolic syndrome. Metab Clin Exp 52:724–730
Lordelo RA, Mancini MC, Cercato C, Halpern A (2007) Hormonal axes in obesity: cause or effect? Arq Bras Endocrinol Metab 51:34–41
Millspaugh JJ, Washburn BE (2004) Use of fecal glucocorticoid metabolite measures in conservation biology research: considerations for application and interpretation. Gen Comp Endocrinol 138:189–199
Möstl E, Palme R (2002) Hormones as indicators of stress. Domest Anim Endocrinol 23:67–74
Möstl E, Maggs JL, Schrotter G, Besenfelder U, Palme R (2002) Measurement of cortisol metabolites in faeces of ruminants. Vet Res Commun 26:127–139
Narayan E, Molinia F, Christi K, Morley C, Cockrem J (2010) Urinary corticosterone metabolite responses to capture, and annual patterns of urinary corticosterone in wild and captive endangered Fijian ground frogs (Platymantis vitiana). Aust J Zool 58:189–197
Palme R (2005) Measuring fecal steroids—guidelines for practical application. Ann N Y Acad Sci 1046:75–80
Palme R, Rettenbacher S, Touma C, El-Bahr SM, Möstl E (2005) Stress hormones in mammals and birds—comparative aspects regarding metabolism, excretion, and noninvasive measurement in fecal samples. Ann N Y Acad Sci 1046:162–171
Rangel-Negrin A, Alfaro JL, Valdez RA, Romano MC, Serio-Silva JC (2009) Stress in Yucatan spider monkeys: effects of environmental conditions on fecal cortisol levels in wild and captive populations. Anim Conserv 12:496–502
Romero LM (2002) Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen Comp Endocrinol 128:1–24
Romero LM (2004) Physiological stress in ecology: lessons from biomedical research. Trends Ecol Evol 19:249–255
Romero LM, Dickens MJ, Cyr NE (2009) The reactive scope model—a new model integrating homeostasis, allostasis, and stress. Horm Behav 55:375–389
Sapolsky RM (2002) Endocrinology of the stress-response. In: Becker JB, Breedlove SM (eds) Behavioral endocrinology. MIT Press, Cambridge, pp 409–450
Seale JV, Wood SA, Atkinson HC, Bate E, Lightman SL, Ingram CD, Jessop DS, Harbuz MS (2004) Gonadectomy reverses the sexually diergic patterns of circadian and stress-induced hypothalamic–pituitary–adrenal axis activity in male and female rats. J Neuroendocrinol 16:516–524
Sheriff M, Dantzer B, Delehanty B, Palme R, Boonstra R (2011) Measuring stress in wildlife: techniques for quantifying glucocorticoids. Oecologia 166:593–605
St. Aubin DJ, Ridgway SH, Wells RS, Rhinehart H (1996) Dolphin thyroid and adrenal hormones: circulating levels in wild and semidomesticated Tursiops truncatus, and influence of sex, age, and season. Mar Mamm Sci 12:1–13
Stead-Richardson E, Bradshaw D, Friend T, Fletcher T (2010) Monitoring reproduction in the critically endangered marsupial, Gilbert’s potoroo (Potorous gilbertii): preliminary analysis of faecal oestradiol-17 beta, cortisol and progestagens. Gen Comp Endocrinol 165:155–162
Terio KA, Marker L, Munson L (2004) Evidence for chronic stress in captive but not free-ranging cheetahs (Acinonyx jubatus) based on adrenal morphology and function. J Wildl Dis 40:259–266
Touma C, Palme R (2005) Measuring fecal glucocorticoid metabolites in mammals and birds: the importance of validation. Ann N Y Acad Sci 1046:54–74
von der Ohe CG, Servheen C (2002) Measuring stress in mammals using fecal glucocorticoids: opportunities and challenges. Wildl Soc Bull 30:1215–1225
Weingrill T, Gray DA, Barrett L, Henzi SP (2004) Fecal cortisol levels in free-ranging female chacma baboons: relationship to dominance, reproductive state and environmental factors. Horm Behav 45:259–269
Wielebnowski N (2003) Stress and distress: evaluating their impact for the well-being of zoo animals. J Am Vet Med Assoc 223:973–977
Wielebnowski N, Watters J (2007) Applying fecal endocrine monitoring to conservation and behavior studies of wild mammals: important considerations and preliminary tests. Isr J Ecol Evolution 53:439–460
Wielebnowski NC, Fletchall N, Carlstead K, Busso JM, Brown JL (2002) Noninvasive assessment of adrenal activity associated with husbandry and behavioral factors in the North American clouded leopard population. Zoo Biol 21:77–98
Woodruff JA, Lacey EA, Bentley G (2010) Contrasting fecal corticosterone metabolite levels in captive and free-living colonial Tuco-Tucos (Ctenomys sociabilis). J Exp Zool Part A Ecol Genet Physiol 313A:498–507
Yildiz B, Azziz R (2007) The adrenal and polycystic ovary syndrome. Rev Endocr Metab Disord 8:331–342
Young KM, Walker SL, Lanthier C, Waddell WT, Monfort SL, Brown JL (2004) Noninvasive monitoring of adrenocortical activity in carnivores by fecal glucocorticold analyses. Gen Comp Endocrinol 137:148–165
Ziegler TE, Scheffler G, Snowdon CT (1995) The relationship of cortisol-levels to social-environment and reproductive functioning in female cotton-top tamarins, Saguinus oedipus. Horm Behav 29:407–424
Acknowledgments
We greatly appreciate the hard work of everyone involved in collecting fecal samples and the assistance provided by participating institutions (Captive: Alaska Zoo, Assiniboine Park Zoo, Brec’s Baton Rouge Zoo, Buttonwood Park Zoo, Cincinnati Zoo & Botanical Garden, Connecticut’s Beardsley Zoo, Dirt Willy’s Game Bird Farm, Feline Conservation Center, Minnesota Zoological Gardens, N.O.A.H. Feline Refuge Center, Pueblo Zoo, Philadelphia Zoo, Salmonier Nature Park, Scovill Zoo, The Wildcat Sanctuary, Toronto Zoo, Utah’s Hogle Zoo, Walk on the Wildside Feline Refuge, Wild Trax Feline Refuge, Wildlife Science Center, Zoo America, and Zoo Sauvage de St. Félicien; Wild: Colorado Division of Wildlife, Maine Department of Inland Fisheries and Wildlife, USFS Rocky Mountain Research Station). Special thanks to Feline Conservation Center, Philadelphia Zoo, Scovill Zoo, and Wildlife Science Center for their participation in the ACTH challenge. Thanks to Astrid Bellem and Jocelyn Bryant for technical support. Funding for this project was provided by the Chicago Zoological Society/Chicago Board of Trade, Purdue University, and PEO International.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I.D. Hume.
Rights and permissions
About this article
Cite this article
Fanson, K.V., Wielebnowski, N.C., Shenk, T.M. et al. Comparative patterns of adrenal activity in captive and wild Canada lynx (Lynx canadensis). J Comp Physiol B 182, 157–165 (2012). https://doi.org/10.1007/s00360-011-0597-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-011-0597-8