Abstract
Background
We evaluated the efficacy and safety of a modified FOLFIRINOX regimen for chemotherapy-naïve patients with metastatic pancreatic cancer.
Methods
Patients with untreated metastatic pancreatic cancer (MPC) received modified FOLFIRINOX (intravenous oxaliplatin 85 mg/m2, irinotecan 150 mg/m2, 5-FU infusion 2400 mg/m2 over 46 h, no bolus 5-FU). The primary endpoints were overall survival and the incidence of grade 3 or higher neutropenia. No patients received prophylactic pegfilgrastim.
Results
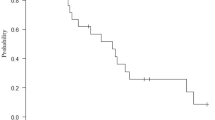
Sixty-nine pts. were enrolled from 39 institutions in Japan. The median overall survival was 11.2 months [95% confidence interval (CI) 9.0–]. The median progression-free survival was 5.5 months (95% CI 4.1–6.7). The response rate was 37.7% (95% CI 26.3–50.2), and the disease control rate was 78.3% (95% CI 66.7–87.3). The incidence of grade 3 or higher neutropenia was 47.8%. Serious adverse events occurred in six patients (8.7%). All AE proportions were less than those in the previous Japanese full-dose phase II study. One patient died due to interstitial pneumonia related to treatment.
Conclusion
This is the first prospective study of modified FOLFIRINOX in Asia. Modified FOLFIRINOX in this study has an improved safety profile with maintained efficacy in MPC without prophylactic pegfilgrastim.


Similar content being viewed by others
References
Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–1825
Mahaseth H, Brutcher E, Kauh J, Hawk N, Kim S, Chen Z, Kooby DA, Maithel SK, Landry J, El-Rayes BF (2013) Modified FOLFIRINOX regimen with improved safety and maintained efficacy in pancreatic adenocarcinoma. Pancreas 42:1311–1315
Okusaka T, Ikeda M, Fukutomi A, Ioka T, Furuse J, Ohkawa S, Isayama H, Boku N (2014) Phase II study of FOLFIRINOX for chemotherapy-naïve Japanese patients with metastatic pancreatic cancer. Cancer Sci 105:1321–1326
Souglakos J, Androulakis N, Syrigos K, Polyzos A, Ziras N, Athanasiadis A, Kakolyris S, Tsousis S, Kouroussis C, Vamvakas L, Kalykaki A, Samonis G, Mavroudis D, Georgoulias V (2006) FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan) vs FOLFIRI (folinic acid, 5-fluorouracil and irinotecan) as first-line treatment in metastatic colorectal cancer (MCC): a multicentre randomised phase III trial from the Hellenic Oncology Research Group (HORG). Br J Cancer 94:798–805
Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, Crinò L, Benedetti G, Evangelista W, Fanchini L, Cortesi E, Picone V, Vitello S, Chiara S, Granetto C, Porcile G, Fioretto L, Orlandini C, Andreuccetti M, Masi G (2007) Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the gruppo oncologico nord ovest. J Clin Oncol 25:1670–1676
Sunakawa Y, Fujita K, Ichikawa W, Ishida H, Yamashita K, Araki K, Miwa K, Kawara K, Akiyama Y, Yamamoto W, Nagashima F, Saji S, Sasaki Y (2012) A phase I study of infusional 5-fluorouracil, leucovorin, oxaliplatin and irinotecan in Japanese patients with advanced colorectal cancer who harbor UGT1A1*1/*1,*1/*6 or *1/*28. Oncology 82:242–248
Stein SM, James ES, Deng Y, Cong X, Kortmansky JS, Li J, Staugaard C, Indukala D, Boustani AM, Patel V, Cha CH, Salem RR, Chang B, Hochster HS, Lacy J (2016) Final analysis of a phase II study of modified FOLFIRINOX in locally advanced and metastatic pancreatic cancer. Br J Cancer 114:737–743
Jones SE, Savin MA, Holmes FA, O’Shaughnessy JA, Blum JL, Vukelja S, McIntyre KJ, Pippen JE, Bordelon JH, Kirby R, Sandbach J, Hyman WJ, Khandelwal P, Negron AG, Richards DA, Anthony SP, Mennel RG, Boehm KA, Meyer WG, Asmar L (2006) Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol 24:5381–5387
Kosaka Y, Rai Y, Masuda N, Takano T, Saeki T, Nakamura S, Shimazaki R, Ito Y, Tokuda Y, Tamura K (2015) Phase III placebo-controlled, double-blind, randomized trial of pegfilgrastim to reduce the risk of febrile neutropenia in breast cancer patients receiving docetaxel/cyclophosphamide chemotherapy. Support Care Cancer 23:1137–1143
de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L, Roessner M, Gupta S, Sartor AO (2010) Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 376:1147–1154
Nozawa M, Mukai H, Takahashi S, Uemura H, Kosaka T, Onozawa Y, Miyazaki J, Suzuki K, Okihara K, Arai Y, Kamba T, Kato M, Nakai Y, Furuse H, Kume H, Ide H, Kitamura H, Yokomizo A, Kimura T, Tomita Y, Ohno K, Kakehi Y (2015) Japanese phase I study of cabazitaxel in metastatic castration-resistant prostate cancer. Int J Clin Oncol 20:1026–1034
von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369:1691–1703
Acknowledgements
We thank all the patients, their families, the investigators, co-medical staffs and all others who participated in the present study.
Funding
This research was supported by the Practical Research for Innovative Cancer Control from the Japan Agency for Medical Research and development, AMED, and in part by The National Cancer Center Research and Development Fund (26-A-4).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Masato Ozaka received the honoraria from Taiho Pharmaceutical, Yakult, Bayer, Pfizer and Novartis. Hiroshi Ishii is a member of a Data and safety Monitoring Board of Ono, and received the honoraria from Eisai, Taiho, Chugai and Astellas. Makoto Ueno received honoraria from Abbott, Eli Lilly, Yakult and AstraZeneca and research funding from Eli Lilly, Kowa, Taiho, Yakult, Daiichi-Sankyo, Kyowa Hakko Kirin, Zeria, Merck Serono and AstraZeneca. Masafumi Ikeda received research funding from received research funding from GlaxoSmithKline, AstraZeneca, Yakult, Ono Pharmaceutical, and Eisai. Nobumasa Mizuno has received research grants from AstraZeneca, Eisai, Merck Serono, MSD, NanoCarrier, Taiho Pharmaceutical, and Zeria Pharmaceutical, and speakers bureau honoraria from Kyowa Hakko Kirin, Novartis, Pfizer, Taiho Pharmaceutical, and Yakult Honsha. Takuji Okusaka has had an editor role, research grant, and honoraria from Novartis; reports research grants and honoraria from Pfizer, Taiho, Bayer, Chugai, Eli Lilly and Company, Yakuruto Honsha, Ono Pharmaceutical, AstraZeneca, Merck Serono, Baxter, Nobelpharma Co.; and research grants from Nippon Boehringer Ingelheim, Dainippon Simitomo Pharma, Eisai Co., OncoTherapy Science Inc., Kyowa Hakko Kirin Co., Shizuoka Industry, Nano Carrier Co., Zeria Pharmaceutical Co. and Glaxo Smith Kline. Junji Furuse received has received honoraria from Taiho Pharmaceutical, Fujifilm, Eisai, Astellas, Yakult, Sumitomo Dainippon, Eli Lilly Japan, and Kyowa Hakko Kirin, and research funding from J-Pharma, Taiho Pharmaceutical, Ono Pharmaceutical, MSD, Janssen and Daiichi Sankyo. The other authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Ozaka, M., Ishii, H., Sato, T. et al. A phase II study of modified FOLFIRINOX for chemotherapy-naïve patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol 81, 1017–1023 (2018). https://doi.org/10.1007/s00280-018-3577-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-018-3577-9