Abstract
Purpose
Curcumin, a major constituent of the spice turmeric, suppresses expression of the enzyme cyclooxygenase 2 (Cox-2) and has cancer chemopreventive properties in rodents. It possesses poor systemic availability. We explored whether formulation with phosphatidylcholine increases the oral bioavailability or affects the metabolite profile of curcumin.
Methods
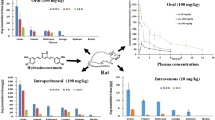
Male Wistar rats received 340 mg/kg of either unformulated curcumin or curcumin formulated with phosphatidylcholine (Meriva) by oral gavage. Rats were killed at 15, 30, 60 and 120 min post administration. Plasma, intestinal mucosa and liver were analysed for the presence of curcumin and metabolites using HPLC with UV detection. Identity of curcumin and metabolites was verified by negative ion electrospray liquid chromatography/tandem mass spectrometry.
Results
Curcumin, the accompanying curcuminoids desmethoxycurcumin and bisdesmethoxycurcumin, and the metabolites tetrahydrocurcumin, hexahydrocurcumin, curcumin glucuronide and curcumin sulfate were identified in plasma, intestinal mucosa and liver of rats which had received Meriva. Peak plasma levels and area under the plasma concentration time curve (AUC) values for parent curcumin after administration of Meriva were fivefold higher than the equivalent values seen after unformulated curcumin. Similarly, liver levels of curcumin were higher after administration of Meriva as compared to unformulated curcumin. In contrast, curcumin concentrations in the gastrointestinal mucosa after ingestion of Meriva were somewhat lower than those observed after administration of unformulated curcumin. Similar observations were made for curcumin metabolites as for parent compound.
Conclusion
The results suggest that curcumin formulated with phosphatidylcholine furnishes higher systemic levels of parent agent than unformulated curcumin.






Similar content being viewed by others
References
Aggarwal BB, Shishioda S (2006) Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol 71:1397–1421
Barzaghi N, Crema F, Gatti G, Pifferi G, Perucca E (1990) Pharmacokinetic studies of IdB 1016, a silybin-phosphatidylcholine complex, in healthy human subjects. Eur J Drug Metab Pharmacokinet 15:333–338
Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Wu MS, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY (2001) Phase 1 clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res 21:2895–2900
Duvoix A, Blasius R, Delhalle S, Schenkenburger M, Morceau F, Henry E, Dicato M, Diederich M (2005) Chemopreventive and therapeutic effects of curcumin. Cancer Lett 223:181–190
Fagerholm U, Sjostrom B, Sroka-Markovic J, Wijk A, Svensson M, Lennernas H (1998) The effect of a drug-delivery system consisting of soybean phosphatidyl choline and medium chain monoacylglycerol on the intestinal permeability of hexarelin in the rat. J Pharm Pharmacol 50:467–473
Garcea G, Jones DJL, Singh R, Dennison AR, Farmer PB, Sharma RA, Steward WP, Gescher AJ, Berry DP 2004) Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br J Cancer 90:1011–1015
Garcea G, Berry DP, Jones DJL, Singh R, Dennison AR, Farmer PB, Sharma RA, Steward WP, Gescher AJ (2005) Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol Biomarkers Prev 14:120–125
Goel A, Boland CR, Chauhan DP (2001) Specific inhibition of cyclooxygenase-2 (Cox-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer Lett 172:111–118
Ireson C, Orr S, Jones DJL, Verschoyle R, Lim CK, Luo JL, Howells L, Plummer S, Jukes R, Williams M, Steward WP, Gescher A (2001) Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E-2 production. Cancer Res 61:1058–1064
Ireson C, Jones DJL, Orr S, Coughtrie MWH, Boocock DJ, Williams ML, Farmer PB, Steward WP, Gescher AJ (2002). Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol Biomarkers Prev 11:105–111
Kawamori T, Lubet R, Steele VE, Kelloff GJ, Kaskey RB, Rao CV, Reddy BS (1999) Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer Cancer Res 59:597–601
Kelloff GJ, Crowell JA, Hawk ET, Steele VE, Lubet RA, Boone CW, Covey JM, Doody LA, Omenn GS, Greenwald P, Hong WK, Parkinson DR, Bagheri D, Baxter GT, Blunden M, Doeltz MK, Eisenhauer KM, Johnson K, Knapp GG, Longfellow DG, Malone WF, Nayfield SG, Seifried HE, Swall LM, Sigman CC (1996) Strategy and planning for chemopreventive drug development: clinical development plans II. J Cell Biochem 63(suppl 26):54–71
Kuttan R, Sudheeran PC, Joseph CD (1987) Turmeric and curcumin as topical agents in cancer therapy. Tumori 73:29–31
Li L, Braiteh FS, Kurzrock R (2005) Liposome-encapsulated curcumin—in vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer 104:1322–1331
Mourao SC, Costa PI, Salgado HRN, Gremiao MPD (2005) Improvement of antischistosomal activity of praziquantel by incorporation into phosphatidylcholine-containing liposomes. Int J Pharm 295:157–162
Osawa T, Sugiyama Y, Inayoshi M, Kawakishi S (1995) Antioxidative activity of tetrahydrocurcuminoids. Biosci Biotechnol Biochem 59:1609–1612
Pan MH, Lin-Shiau SY, Lin JK (2000) Comparative studies on the suppression of nitric oxide synthase by curcumin and its hydrogenated metabolites through down-regulation of IκB kinase and NFκB activation in macrophages. Biochem Pharmacol 60:1665–1676
Plummer SM., Holloway KA, Manson MM, Munks RJL, Kaptein A, Farrow S, Howells L (1999) Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF- kappa B activation via the NIK/IKK signalling complex. Oncogene 18:6013–6020
Rao CV, Rivenson A, Simi B, Reddy BS (1995) Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolics compound. Cancer Res 55:259–266
Shao Z-M, Shen Z-Z, Liu C-H, Sartippour MR, Go VL, Heber D, Nguyen M (2002) Curcumin exerts multiple suppressive effects on human breast carcinoma cells. Int J Cancer 98:234–240
Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, Marczylo TH, Morgan B, Hemingway D, Plummer SM, Pirmohamed M, Gescher AJ, Steward WP (2004) Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res 10:6847–6854
Sugiyama Y, Kawakishi S, Osawa T(1996) Involvement of the beta-diketone moiety in the antioxidative mechanism of tetrahydrocurcumin. Biochem Pharmacol 52:519–525
Surh YJ (2003) Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer 3:768–780
Uehara SI, Yasuda I, Akiyama K, Morita H, Takeya K, Itokawa H (1987) Diarylheptanoids from the rhizomes of curcuma xanthorrhiza and alponia officinarum. Chem Pharm Bull 35:3298–3304
Workman P, Twentyman P, Balkwill F, Balmain A, Chaplin D, Double J, Embleton J, Newell D, Raymond R, Stables J, Stephens T, Wallace J (1998) United Kingdom Co-ordinating Committee on Cancer Research (UKCCCR) guidelines for the welfare of animals in experimental neoplasia (second edition). Br J Cancer 77:1–10
Acknowledgments
This study was supported by programme grant G0100874 from the UK Medical Research Council. The authors thank staff of the Biomedical Services Unit, University of Leicester, for animal husbandry and Indena SpA, Milan, Italy, for the provision of formulated curcumin.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marczylo, T.H., Verschoyle, R.D., Cooke, D.N. et al. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother Pharmacol 60, 171–177 (2007). https://doi.org/10.1007/s00280-006-0355-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-006-0355-x