Abstract
This is a position paper about the therapeutic effects of locally applied free IL-2 in the treatment of cancer. Local therapy: IL-2 therapy of cancer was originally introduced as a systemic therapy. This therapy led to about 20% objective responses. Systemic therapy however was very toxic due to the vascular leakage syndrome. Nevertheless, this treatment was a break-through in cancer immunotherapy and stimulated some interesting questions: Supposing that the mechanism of IL-2 treatment is both proliferation and tumoricidal activity of the tumor infiltrating cells, then locally applied IL-2 should result in a much higher local IL-2 concentration than systemic IL-2 application. Consequently a greater beneficial effect could be expected after local IL-2 application (peritumoral = juxtatumoral, intratumoral, intra-arterial, intracavitary, or intratracheal = inhalation). Free IL-2: Many groups have tried to prepare a more effective IL-2 formulation than free IL-2. Examples are slow release systems, insertion of the IL-2 gene into a tumor cell causing prolonged IL-2 release. However, logistically free IL-2 is much easier to apply; hence we concentrated in this review and in most of our experiments on the use of free IL-2. Local therapy with free IL-2 may be effective against transplanted tumors in experimental animals, and against various spontaneous carcinomas, sarcomas, and melanoma in veterinary and human cancer patients. It may induce rejection of very large, metastasized tumor loads, for instance advanced clinical tumors. The effects of even a single IL-2 application may be impressive. Not each tumor or tumor type is sensitive to local IL-2 application. For instance transplanted EL4 lymphoma or TLX9 lymphoma were not sensitive in our hands. Also the extent of sensitivity differs: In Bovine Ocular Squamous Cell Carcinoma (BOSCC) often a complete regression is obtained, whereas with the Bovine Vulval Papilloma and Carcinoma Complex (BVPCC) mainly stable disease is attained. Analysis of the results of local IL-2 therapy in 288 cases of cancer in human patients shows that there were 27% Complete Regressions (CR), 23% Partial Regressions (PR), 18% Stable Disease (SD), and 32% Progressive Disease (PD). In all tumors analyzed, local IL-2 therapy was more effective than systemic IL-2 treatment. Intratumoral IL-2 applications are more effective than peritumoral application or application at a distant site. Tumor regression induced by intratumoral IL-2 application may be a fast process (requiring about a week) in the case of a highly vascular tumor since IL-2 induces vascular leakage/edema and consequently massive tumor necrosis. The latter then stimulates an immune response. In less vascular tumors or less vascular tumor sites, regression may require 9–20 months; this regression is mainly caused by a cytotoxic leukocyte reaction. Hence the disadvantageous vascular leakage syndrome complicating systemic treatment is however advantageous in local treatment, since local edema may initiate tumor necrosis. Thus the therapeutic effect of local IL-2 treatment is not primarily based on tumor immunity, but tumor immunity seems to be useful as a secondary component of the IL-2 induced local processes. If local IL-2 is combined with surgery, radiotherapy or local chemotherapy the therapeutic effect is usually greater than with either therapy alone. Hence local free IL-2 application can be recommended as an addition to standard treatment protocols. Local treatment with free IL-2 is straightforward and can readily be applied even during surgical interventions. Local IL-2 treatment is usually without serious side effects and besides minor complaints it is generally well supported. Only small quantities of IL-2 are required. Hence the therapy is relatively cheap. A single IL-2 application of 4.5 million U IL-2 costs about 70 Euros. Thus combined local treatment may offer an alternative in those circumstances when more expensive forms of treatment are not available, for instance in resource poor countries.
Similar content being viewed by others
Systemic IL-2 therapy of cancer
Rosenberg and co-workers were the first to treat cancer with IL-2. They showed that IL-2 renders white blood cells cytotoxic in vitro. These cells were coined Lymphokine Activated Killer cells (LAK cells). Rosenberg and co-workers treated transplanted lung metastases in mice. Injection of IL-2 plus LAK cells clearly reduced the number of metastases in the lung [82]. Next these authors treated 25 consecutive patients with different types of advanced cancer for whom no effective treatment was available. In 11 patients, objective responses were obtained after treatment with IL-2 and LAK cells [81]. Obviously this paper attracted worldwide attention, as it was a breakthrough in immunological treatment of cancer. In further studies, systemic application of IL-2 with or without LAK cells appeared useful in patients with metastasized renal cell carcinoma and metastasized melanoma. In both conditions about 20% objective responses were obtained; that is about 6% complete and about 14% partial tumor regressions [57]. Repeated cycles of systemic IL-2 administration were required to achieve systemic tumor-inhibitory effects. Grande et al. [46] recently reviewed the therapeutic effects of systemic IL-2 therapy.
Systemic IL-2 application required bolus injections of IL-2 given every 8 h at a dose of 105 IU/kg body weight for at least 5 days. These high doses were very toxic, as systemic IL-2 therapy induces a generalized vascular leakage syndrome [7, 83]. In addition the preparation and application of LAK cells was cumbersome indeed. Supposing that the tumor infiltrating leucocytes have to be stimulated by IL-2, we and other groups decided to focus on local IL-2 therapy, that is IL-2 application at the site of the tumor (peritumoral = juxtatumoral, intratumoral, intra-arterial, intracavitary infusion, or inhalation). Since local IL-2 application requires smaller doses of IL-2 than systemic treatment, less complications were expected. Locally applied relatively small doses of IL-2 leads to much higher IL-2 concentrations at the site of the tumor and to much lower concentrations elsewhere in the body.
Conclusion
The development of systemic IL-2 therapy was a break-through in cancer immunotherapy. We supposed that the drawbacks of systemic IL-2 application might be overcome by local IL-2 application.
Effectiveness of local versus systemic IL-2 therapy
Subject
The therapeutic effectiveness of local versus systemic IL-2 application.
Data
Silagi et al. [86] studied mice with melanoma or sarcoma using combined cyclosphosphamide and IL-2. Cyclophosphamide was always applied systemically. Cyclophosphamide alone had no therapeutic effect but combined treatment of cyclophosphamide and IL-2 showed a synergistic effect. When either tumor was implanted s.c. at day 0, and IL-2 treatment was given at the site of the tumor beginning 1–3 days later, 87–100% of the mice were cured. Only 35–50% were cured when IL-2 was administered i.p. Conversely, with i.p. treatment of i.p. tumors, 60–83% of the mice were tumor-free on day 50, as compared with only 17% with s.c.treatment.
Vaage [94] tested the therapeutic effects of 12 daily injections of 100–300,000 U of human IL-2 against the syngeneic, immunogenic mammary carcinoma MC2 implanted s.c. into C3H/He mice. A local therapeutic effect was observed after injecting tumors even with doses as low as 300 U of IL-2 per injection. Systemic IL-2 treatment however required at least 5,000 U per injection for obtaining discernable results.
Belardelli et al. [10] treated mice with s.c. transplanted highly metastatic Friend leukemia, non-metastatic Friend leukemia, RBL-5 lymphoma, and HeJ16 fibrosarcoma. In all these tumor models, peritumoral injections of IL-2 were more effective in inhibiting tumor growth than systemic treatment.
Anderson et al. [2] studied the therapeutic effects of IL-2 in C57BL/6 mice with MCA-106 sarcoma pulmonary metastases. When mice were treated once daily with free IL-2 on days 5, 6, and 7 after tumor inoculation, the intrathoracic route was superior to the i.p. or s.c. routes.
Maas et al. [68] treated DBA/2 mice bearing i.p. and s.c. SL2 lymphoma. If IL-2 was injected i.p., then in 70% of the mice i.p. tumors regressed completely, and in 50% tumors regressed completely both i.p. and s.c. When mice had only a s.c. growing tumor and IL-2 was injected i.p., then in only 7% of the mice tumors regressed completely.
Dubinett et al. [33] transplanted s.c. Line 1 alveolar carcinoma cells in Balb/c mice. On the third day following tumor implantation, mice received injections of IL-2 twice daily, either by i.p. or intratumoral injection, 5 days/week for 3 weeks. Intratumoral injection of IL-2 significantly reduced the tumor volume, increased the median survival time, and resulted in a 23.5% cure rate. However no long-term survivors were among the i.p. treated mice.
Jacobs et al. [55] investigated human patients with nasopharyngeal carcinoma. Local IL-2 treatment was combined with standard irradiation therapy. Sixty three % of the patients showed a disease free survival during the next 5 years, whereas only 8% of the controls treated with irradiation alone were still disease free after 5 years. Interestingly, these results contrast with those of Chi et al. [23] who applied single modality systemic IL-2 therapy. They concluded that no response was observed. So, apparently, local IL-2 application is more effective than systemic application. It is important that these results of Jacobs et al. and Chi et al. were obtained in spontaneously occurring tumors, not with transplanted tumors.
Local IL-2 should even preferably be given intratumorally as IL-2 application adjacent to the tumor is far less effective. Jacobs et al. [56] compared the effect of peritumoral and intratumoral IL-2 therapy in mice with s.c. transplanted SL2 lymphoma. Intratumoral IL-2 was significantly more effective than peritumoral IL-2. Krastev et al. [61] treated 16 patients with various gastrointestinal tumors with intratumoral and/or intraperitoneal IL-2 therapy. Six patients had a clinical response. All six belonged to the group of seven patients who received intratumoral therapy. No objective responses were obtained in patients treated only with intraperitoneal IL-2.
In an early review Bernsen et al. already concluded that locoregional IL-2 treatment was more effective than systemic treatment [13]. Since then an overwhelming amount of research has corroborated this conclusion.
Conclusion
The data show that local IL-2 therapy and in particular intratumoral IL-2 application is more effective than systemic IL-2 therapy.
Local IL-2 therapy leads to systemic therapeutic effects even curing metastatic disease
Subject
Obviously a major problem of cancer therapy is inducing regression of metastases. Immunotherapy of cancer is an attractive concept, as systemic immunity may indeed cure metastases. Consequently, many groups have concentrated on the systemic therapeutic effects of local IL-2 therapy.
Data
Maas et al. [67] have shown that i.p. injections of IL-2 can cure DBA/2 mice with a large burden of i.p. transplanted and greatly disseminated SL2 lymphoma. This implied that tumor metastases can be successfully treated with local IL-2 therapy. In more detailed studies SL2 tumor cells were injected in mice both i.p. and s.c. on the flank resulting in i.p. and s.c. tumors [67]. About 50% of the mice treated i.p with IL-2 rejected both the i.p. tumor and the large distant s.c. tumor. In contrast, similar i.p. treatment cured only 7% of the mice bearing only a s.c. SL2 tumor. Thus, it was shown that IL-2 can induce systemic tumor rejection when injected at the site of tumor growth. This SL2 tumor rejection was specific, as mice that were rejecting i.p. and s.c. SL2 lymphoma did not reject P815 mastocytoma.
Vaage [95] tested the therapeutic effects of IL-2 against intramammary implants of an immunogenic, syngeneic C3H mammary carcinoma. Peritumoral injected IL-2 had almost equal local and systemic therapeutic effects, whereas systemically injected IL-2 was not therapeutically effective at all.
Immunity also explains the long lasting absence (>54 months) of bladder carcinoma recurrences in two IL-2 treated patients who previously had a 7 and 11 years’ history of recurrent bladder cancer [29].
Van Es et al. [96] showed that peritumoral injections of IL-2 in a transplanted rabbit carcinoma model can induce complete regression of the treated tumors as well as untreated contralateral tumors in four out of 12 rabbits. Also metastases in the draining regional lymph nodes of both treated and untreated primary tumors regressed in three of these animals. So, local treatment of a tumor led to systemic effects curing untreated tumors at a distant site as well as metastases in the draining lymph nodes of both the treated and the untreated tumors. A second challenge with tumor cells of the cured animals was rejected.
Jacobs et al. [56] transplanted in mice tumors at two different sites. Rejection of the intratumoral IL-2 treated tumors was stronger than rejection of the untreated tumors.
Systemic immunity is also likely in patients with nasopharyngeal carcinoma treated with radiotherapy and intratumoral IL-2 application [55]. Addition of IL-2 to the standard radiotherapy reduced the number of loco regional and distant recurrences.
In line with these observations is the finding that local or generalized effector dysfunction of the immune system can be reversed by IL-2 exposure in patients with advanced cancer [70].
Conclusion
Local IL-2 therapy can also cause systemic therapeutic effects, probably due to immune reactivity [68, 96].
Therapeutic effects of local IL-2 therapy against transplanted tumors in laboratory animals (Tables 1, 2)
Subject
For studying the potential therapeutic effects of anticancer drugs one has to start in animal models using transplanted tumors, as well-performed and well-interpreted studies in animals have predictive value regarding the therapeutic effectiveness of a drug in human cancer patients [32].
Data
As early as in 1983, Bubenik and co-workers [18] established that peritumoral injections of rat lymphoid IL-2 suppressed or markedly inhibited the growth of methylcholanthrene-induced sarcomas in syngeneic mice. An equally effective inhibition of murine sarcoma transplants in syngeneic recipients could be obtained with crude lymphoid rat IL-2, with purified IL-2 of murine lymphoid origin, and with molecularly homogeneous human recombinant IL-2 [16–18].
Maas et al. [67] injected DBA/2 mice i.p. with SL2 cells and 10–14 days later these mice were treated with i.p. IL-2 injections. At the time of IL-2 injection the transplanted SL2 tumor had greatly expanded by growth, infiltration and metastasis. A mouse of 25 gram developed in 10 days a tumor load of at least 5 g, about 5 × 109 tumor cells. Nevertheless about 25% of these mice were cured by IL-2. This was a very important step forward in immunotherapy of cancer. The therapeutic effects were dose-dependent. These data were confirmed in more elaborated studies by Bernsen et al. [12] and by Everse et al. [37].
Tables 1 and 2 summarize the therapeutic results of local IL-2 application to transplanted tumors. Table 1 displays the results obtained by other groups and Table 2 results by our group. Both Tables show that local IL-2 therapy may be effective in a broad range of tumor types such as carcinomas [6, 33, 42, 50, 58, 64, 72, 73, 87, 94, 96], sarcomas [10, 16–18, 86], a myeloma [69], lymphomas [10, 66, 67], leukemia [10], a mastocytoma [68], and HPV associated tumor [21].
Obviously not all cancers are sensitive to IL-2 therapy. Our group studied the effect of local IL-2 therapy in 19 models of transplanted tumors (Table 2). In 15 models positive therapeutic results were obtained. IL-2 applied in breast cancers was only moderately effective. In four models no therapeutic effects were obtained, namely murine 5D04 stomach carcinoma [27], murine MOT teratoma [14], murine EL4 lymphoma [27] and murine TLX9 lymphoma [27]. Why some tumors are sensitive to local IL-2 therapy and other tumor models do not show any response, remains hitherto an enigma. The data summarized in Tables 1 and 2 demonstrate that local IL-2 therapy has the capability to destroy tumor cells and to cure the hosts.
An animal model using transplanted tumors has predictive value for the therapeutic effect in human cancer if the model tumor comprises more than 1% of the body weight of the host and if this tumor is metastasized [32]. Eight models mentioned in Table 2 fulfil these requirements, viz. the models marked in column 4 with +. So, the positive therapeutic results obtained with these models predict that local IL-2 therapy can be therapeutically effective in human patients with metastatic cancer.
Conclusion
In models with transplanted tumors in laboratory animals there is overwhelming evidence showing the therapeutic effect of local application of free IL-2. This therapy is effective against a broad range of tumors. In addition the magnitude of the therapeutic data (for instance [67]) suggests that this form of therapy can also induce objective therapeutic responses in human cancer patients.
N.B. Not every transplanted tumor or tumor type is sensitive to local IL2 application. For instance transplanted EL4 lymphoma or TLX9 lymphoma are not sensitive in our hands (Table 2).
Local IL-2 tumor treatment in veterinary patients (Table 3)
Subject
Positive results in veterinary patients with spontaneous cancer are an important intermediate between experiments with transplanted cancer in laboratory animals and clinical application in human cancer cases. If therapeutic effects are positive in well-performed, well-interpreted experiments with transplanted cancer in laboratory animals as well as in spontaneous cancer in veterinary patients, then one can be almost certain that such a therapy will also be effective in human cancer patients.
Data
Table 3 summarizes all published studies that we know on local IL-2 application in veterinary cancer patients.
Bovine Ocular Squamous Cell Carcinoma (BOSCC)
BOSCC originates in the cornea, the third eyelid (membrana nictitans), the lower or the upper eyelid. Ultimately the tumor covers the whole eye. It also metastasizes to the draining lymph nodes. This tumor occurs frequently in tropical countries with intense solar radiation, particularly at high altitudes. BOSCC is for various reasons a very useful veterinary tumor model: The tumor is readily visible and can be directly treated with peritumoral or intratumoral IL-2 injection in field studies.
In The Netherlands BOSCC is a very rare disease. So our first tests of IL-2 sensitivity of BOSCC were performed in only five cows with BOSCC [84]. The results showed that BOSCC can be sensitive to local IL-2 therapy. In Zimbabwe about 10% of the cows are affected by BOSCC. This allows large-scale studies [31]. Added to this BOSCC causes an enormous economical burden. In our most extensive study in Zimbabwe [86] we treated 174 BOSCC cases with tumor areas ranging from 20 to 2,800 mm2. Peritumoral injections of various doses of IL-2 were applied during 2 × 5 days. Nine months after treatment, the daily doses of 5 × 103, 2 × 104, 2 × 105, 5 × 105, 1 × 106, 2 × 106 U IL-2 had induced complete tumor regression in 82, 81, 56, 15, 44, and 35% of the animals, respectively. In the control animals the tumors had completely regressed in only 14% of the cases. After 20 months the comparable figures were 55, 52, 58, 50, 69 and 52%, respectively, and there had been no change in the control group. The tumors on the third eyelid and limbus were the most responsive [89]. Similar results were obtained in other studies [30, 90].
Even large BOSCC tumors of up to 66 mm can regress completely by local IL-2 therapy [31].
Bovine Vulval Papilloma and Carcinoma Complex (BVPCC)
BVPCC is a common disease in Bos taurus breeds of cattle kept at high altitude with high levels of solar radiation in Africa [19, 51]. It also occurs in other countries and continents with similar high levels of solar radiation. Burdin [19] originally described the pathogenesis of this neoplasm. Hill et al. [51] adapted this description to develop a useful system for clinical staging of the tumors. In Zimbabwe BVPCC occurs in about 10% of the cattle. It causes much animal suffering. In addition BVPCC usually proceeds to a more advanced stage. So BVPCC forms a real economical burden for the farmer and on a national scale.
Twenty three papillomas and carcinomas of the bovine vulva were treated with local IL-2 therapy. Sixteen partial remissions and three complete remissions add to a tumor reduction in 83% of the treated cows [Stewart et al. to be published]. Remissions were striking in papillomas with a massive lymphocytic infiltrate in particular in those epithelial areas that showed marked dysplasia or (pre-)malignant changes.
Sarcoids
Sarcoids are fibro-epithelial skin tumors of horses, donkeys, and mules. Infiltrative growth is prominent but they seldom metastasize. After surgical removal they usually recur. Sarcoids were treated by intratumoral IL-2 injections for 5 or 10 days. There were 36 and 50% objective responses, respectively after 12 months [88].
Fibrosarcomas in dogs
Tumors often occur in cats and dogs as owners care for pets even into advanced age as members of the family. Like ageing humans they show a large variety of tumors in their later years. Preliminary data demonstrated that fibrosarcomas in dogs are sensitive to local IL-2 therapy [100].
Conclusion
Local IL-2 therapy can be effective against spontaneous veterinary tumors. Local IL-2 application has an enormous economical impact as BOSCC as well as BVPCC occur in about 10% of cattle in Zimbabwe and probably also in other tropical countries. Local IL-2 treatment of BOSCC leads to CR in the majority of the cases, and led to tumor reduction in the majority of BVPCC cases.
For ethical reasons specific immunity cannot be tested in veterinary patients. However specificity and systemic effects have been shown in mice [68] and rabbits [96] with transplanted tumors. Effective local IL-2 treatment greatly improves the quality of life by reducing suffering. These therapeutic effects in veterinary cancer patients make a strong case for the development and acceptance of local IL-2 therapy with free IL-2 in human cancer patients.
Results of local IL-2 tumor treatment in human cancer patients (Table 4)
Subject
The final step in the experimental chain is the local application of free IL-2 to human cancer. Many research groups have pioneered in this field. We now summarize and discuss the results.
Data
Basal cell carcinoma
Kaplan et al. [59] treated basal carcinoma of the skin. A total of 12 tumors were treated in eight patients. Overall response rates were : complete response in 8 of 12 treated tumors, partial response in 3 out of 12 treated tumors, stable disease with no improvement in one tumor site.
Bladder carcinoma
Pizza et al. [79] obtained tumor regressions after intralesional injections of IL-2 in bladder cancer. Repeated injections of IL-2 under cystoscopic control resulted in complete regression of the tumor in three out of six patients and in partial regression of another three patients.
Huland and Huland [53] obtained histologically confirmed complete remission lasting more than 6 months in one out of five patients with urinary bladder carcinoma after continuous IL-2 perfusion of the bladder for 5 days.
Gomella et al. [44, 45] treated 14 patients with superficial bladder carcinoma. Patients were treated first with transurethral resection leaving a marker lesion, followed by intravesical IL-2 instillation. There were three complete responses, one lasting more than 9 months.
Ferlazzo et al. [40, 41] treated superficial bladder cancer cases with intravesical infusions of IL-2. This gave similar clinical results as obtained by vesical instillation with BCG after transurethral resection.
Den Otter et al. [26, 29] treated patients with recurrent bladder carcinoma stage T1, grades 1 to 2 with incomplete transurethral resection leaving a marker tumor of 0.5–1.0 cm. Two days after resection IL-2 was instilled for 2 h. Patients were asked to turn over regularly in order to ensure maximal exposure of IL-2 to the bladder wall. This procedure was repeated on five consecutive days. Two months later the effect was measured by cystoscopy examination. In eight out of ten patients the marker tumor had regressed completely. Four patients were still tumor free after 30 to 54 months. In one patient with a 7-years’ history of bladder cancer requiring 23 cystoscopies, the marker had only partially regressed after 2 months. After removal of the remainder of the marker this patient was tumor free during the follow-up of 54 months. Also, a patient with an 11 years’ history of recurrent bladder cancer remained tumor free during the whole follow-up period [29]. The finding that these patients remained tumor free for >54 months suggests that these patients were (locally) immune to the tumor after tumor regression.
Grasso et al. [47] treated 27 patients with transitional bladder carcinoma Ta/T1-G1–2 with intravesical instillations of IL-2 for 1 h during 5 days. After 2 months none of the lesions had disappeared or were clearly reduced. This result seems to contrast with the findings by Den Otter et al. [26, 29]. The different treatment protocols may be essential: Den Otter et al. started with an incomplete TUR followed by IL-2 instillations, whereas Grasso et al. did not perform a TUR before IL-2 instillation. TUR causes tumor cell damage, which may induce tumor immunity. Interestingly, Grasso et al. obtained 33.3% relapses after a median follow-up of 12 months; this contrasted to the restrospective analysis in which the historical recurrence rate per year was 95%.
Gastrointestinal tumors
Shirai et al. [85] treated five patients with hepatocellular carcinoma with intratumoral IL-2 injections. In two patients 32 and 57% tumor regression was observed.
Krastev et al. [61] treated patients with different forms of stage III and IV gastrointestinal malignancies (primary or metastatic) for whom no further treatment options were available. With locoregionally applied IL-2 a modest but clinically worthwhile improvement was obtained in six out of 16 patients; remarkably these six all belonged to the group of seven patients that were treated with intratumoral IL-2.
Melanoma
Radny et al. [80] treated patients with skin and soft-tissue melanoma metastases with intralesional injection of IL-2. A total of 24 patients with AJCC stage III or IV melanoma and single or multiple skin and soft-tissue metastases were included. IL-2 was administered intralesionally into all cutaneous and soft-tissue metastases accessible from the skin, 2–3 times weekly, over 1–57 weeks. Response evaluation was confined to the intralesionally treated tumors. CR of the treated metastases was achieved in 15 patients, the longest remission lasting 38 months to date. In five patients a PR was achieved and in a further three PD (one patient was not assessable). A total of 245 metastases were treated. There was CR in 209 (85%), and PR in 21 (6%). The therapy was generally well tolerated; the observed adverse events were mainly of grade 1–2 severity.
Pfohler and coworkers [78] treated two patients with multiple cutaneous metastases of malignant melanoma with intra and perilesional application of interleukin-2 and achieved complete regression of these metastases.
Mesothelioma
Goey et al. [43] treated patients with pleural mesothelioma stage I-IIA with continuous daily intrapleural infusion of IL-2. PR occurred in four out of 21 evaluable patients with a median time to progression of 12 months (range 5–37). SD occurred in seven patients with a median time to progression of 5 months (range 2–7). There were no CRs. The median overall survival time was 15.6 months (range 3.0–43).
Astoul et al. [5] treated 22 patients with malignant pleural mesothelioma. The response rate was evaluated 36 days after treatment. There were one CR, 11 PR, three SD, and seven PD. The median survival time of responders differed significantly from that of the non-responders (28 versus 8 months).
Castagneto et al. [22] treated 31 consecutive patients with unresectable malignant pleural mesothelioma with pleural effusion with intrapleural instillation of IL-2. In 90% of the patients there was no further or minimal asymptomatic pleural fluid collection. Median overall survival was 15 months whereas the expected survival range of patients with involvement of the visceral pleura is 9–12 months.
Krastev et al. [62] treated a patient with a large abdominal mesothelioma with intratumoral IL-2 injections and IL-2 instillation in the peritoneal cavity. The tumor regressed completely; the patient was cured and is still healthy and working 6 years after publication.
Neoplastic effusions
Masotti et al. [71] treated neoplastic pleural effusions in 21 patients with non-small cell lung cancer with intrapleural administration of IL-2. CR was obtained in seven patients and PR in six patients.
Lissoni et al. [65] treated 14 patients with neoplastic effusions from a variety of solid tumors. There were four CR and six PR with a median duration of 4 months.
Castagneto et al. [22] treated pleural effusions of mesothelioma as described in the previous section.
Ovarian carcinoma
Edwards et al. [34] treated patients with ovarian carcinoma with infusions of IL-2. Eligibility criteria included six or more courses of prior platinum-based chemotherapy and laparotomy-confirmed persistent or recurrent ovarian cancer. Among 35 assessable patients, there were six laparotomy-confirmed CRs and three PRs. The median survival time of the cohort was 13.7 months and the overall 5-year survival probability was 13.9%. For the nine patients who demonstrated responses, the median survival time had not been reached at the time of publication (range 27 to 90+ months).
Taylor et al. [91] treated patients with advanced ovarian cancer with intraperitoneal IL-2. Nine out of 17 patients showed an objective response.
Renal cell carcinoma (lung metastases)
Huland et al. [52] introduced IL-2 inhalation therapy for lung metastases of renal cell carcinoma. Progressive pulmonary metastases responded dramatically in 15% of the patients for a median of 15.5 months and were stabilized in 55% of patients for a median of 6.6 months. The overall median response duration was 9.6 months. Median survival was 11.8 months; expected survival according to risk analysis was 5.3 months [52].
The PortugeseSpanish Inhaled IL-2 Group [36] studied the effect of inhaled IL-2 on pulmonary metastases of renal cell carcinomas. They found 13.7% Objective Responses (OR), a median progression free survival of 8.6 months and an overall survival of 23 months.
Quantification of therapeutic data
Therapeutic data of Table 4 were further analyzed regarding CR, PR, SD, and PD. This analysis was possible in 288 cancer patients treated with locally applied IL-2. There were 27% CR, 23% PR, 18% SD, and 32% PD. These data may be too optimistic as positive results are published more frequently than negative results.
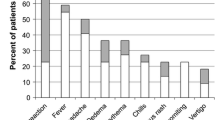
Toxic effect of local IL-2 application
Table 5 shows the toxic effect of local IL-2 application to cancer patients. There were 24 papers with an abstract mentioning toxic side effects. In 20 papers there were no or minor side effects, one paper [41] reports some side effects, and three papers [22, 34, 43] mention more serious side effects. The latter papers have in common that very high IL-2 doses (see Table 5) were used for intrapleural, intraperitoneal or subcutaneous administration. In essence these massive doses act in a pseudo-systemic manner. Much of these massive doses of IL-2 will be absorbed and as a consequence will give generalized effects similar to systemic treatment. Hence, toxicity mentioned in the latter three papers form the exception to the rule that local IL-2 application causes no or minimal side effects. In reference [43] side effects are due to the very high daily doses of 36 × 106 IU IL-2, as this was a Phase I-IIA study with escalating doses of IL-2. In this case IL-2 treatment may give systemic toxicity complications. In paper [22] patients were treated with repeated intrapleural instillations with 9 × 106 IU twice weekly for 4 weeks; in addition, in non-progressing patients 3 × 106 IU IL-2 were administered s.c. thrice weekly for up to 6 months. Obviously such high intrapleural doses are readily resorbed and reach the general circulation. Hence the toxicity (grade 3 fever and grade 3 cardiac toxicity) in 7/31 patients is not surprising. In paper [34] dose-limiting toxicity was seen in patients treated with 7-days’ infusions with the maximum tolerated dose; obviously there was toxicity per definition.
Dose–response
Figure 1 shows the total applied doses of IL-2 and the percentage of objective responses. Results suggest that (a) good therapeutic results can be obtained with low doses of IL-2 (103 to 105 IU); (b) that there is no dose-response effect in the range of total dose of 106 up to 109 IU IL-2. Of course, in this figure there are only a few data for different tumors treated slightly different by different groups. More detailed analysis with homogeneous groups of mice with transplanted SL2 lymphoma in DBA/2 mice showed a dose response effect in the dose range of 5,000–200,000 IU IL-2 given on five consecutive days [12]. It is not surprising that this was not reproduced with the very heterogeneous data in Fig. 1. Nevertheless these data of Fig. 1 are remarkable as total doses of 106–109 IU of IL-2 can result in 20–100% objective responses. We have analysed whether high and low percentages of objective responses are obtained with sensitive and non-sensitive tumor types, respectively. This seems not to be the case as bladder carcinoma, mesothelioma, and ovarian carcinoma were reported as sensitive (>60% OR) as well as non-sensitive (<60% OR) (Table 4; Fig. 1). Another hypothesis is offered by the work of Jacobs et al. [55]. He found that intratumoral IL-2 application is more effective than peritumoral IL-2 application. We therefore further analyzed whether the different therapeutic effects can be ascribed to differences in the localization of IL-2 application. Also this was not the case. Another possibility is that intratumoral IL-2 application leads to more intratumoral edema formation/leakage causing hemodynamic stagnation and additional tumor necrosis. This may stimulate the antitumor response and consequently lead to superior therapeutic effects. Obviously this hypothesis is difficult to study in human cancer patients for logistical and ethical reasons. A more simple explanation of the absence of a dose-response effect is that different tumors have different sensitivity for local IL-2 therapy, e.g., due to different tumor associated antigenicity. Those that are well-responsive can be cured by lower doses, those that are less responsive are (automatically) treated with higher doses. Hence it is impossible to evaluate dose-response effects using different tumors.
% Objective response versus total applied dose of IL-2. The data are derived from the abstracts of the papers mentioned in Table 4
Conclusions
-
Local IL-2 therapy can be effective against a variety of human tumors.
-
In some studies results suggest that metastases are cured and that (systemic) immunity is obtained [29].
-
There is no obvious dose–response with regard to therapeutic effects.
-
Good therapeutic effects can be obtained with total doses of 103 to 105 IU IL-2; these doses cause no or negligible toxic effects. High total IL-2 doses of 108 IU and higher may exert toxic side effects as are described in systemic treatment.
Combined therapy modalities (Table 6)
Subject
Combination of various therapeutic modalities, like surgery, irradiation, chemotherapy, hormone therapy, etc., are standard in tumor treatment. For this reason several groups have also used local IL-2 therapy with free IL-2 combined with other treatment modalities.
Data
Surgery
Ziekman [100] described cases of incomplete surgical removal of a tumor in veterinary patients, followed by intra-operative local IL-2 application. This led to complete tumor regression for instance in dogs with fibrosarcomas.
IL-2 instillation in the bladder to treat bladder tumors seems only effective after a preceeding TUR (see above).
Radiotherapy
Everse et al. [38] treated mice with s.c. growing tumors with radiotherapy combined with IL-2 therapy. The combined therapy was more effective than radiotherapy or IL-2 therapy alone. Similar results were obtained by Jűrgenliemk-Schulz et al. [58].
Early nasopharyngeal carcinoma is not clinically apparent and hence these tumors are often diagnosed in an advanced stage. The standard treatment is irradiation, often complemented with chemotherapy. In The Netherlands usually WHO type 1 nasopharynx carcinoma occurs. Type 1 is very therapy resistant. That is, only about 10% of the patients treated with irradiation have a 5 years’ tumor free survival [55]. In this study tumors of 10 patients were irradiated daily for 7 weeks. In weeks 2, 4, and 6 the tumors were (transnasally) injected with low doses of IL-2 from Monday through Friday. The 5 years’ tumor free survival was 63% [55].
Cytokine therapy
Belardelli et al. [10] described that combined treatment with IL-1 beta and IL-2 produced a synergistic antitumor effect: 60% of mice injected with highly metastasized Friend Leukemia Cells survived. T cells appeared to be essential for IL-1/IL-2 therapy.
Chemotherapy
Enk et al. [35] treated melanoma patients with pulmonary metastases with dacarbazine and concurrent inhalation of IL-2. The patients’ condition previously had progressed on chemotherapy, predominantly on dacarbazine based regimens. Five of the 27 patients experienced a complete pulmonary remission. Eight patients achieved a partial pulmonary remission, and five patients experienced stabilization of the disease. Four of the five patients with a complete response and seven of the eight patients with a partial response were previously treated with dacarbazine and progressed. The complete responses all persisted during a follow-up of 12 months, whereas patients with partial responses or stable disease progressed when IL-2 was discontinued.
Silagi et al. [86] found synergism between cyclophosphamide and IL-2 in the treatment of mice with sarcoma or melanoma.
Bernsen et al. [14] treated mice bearing Murine Ovarian Teratocarcinoma (MOT) with cis-platinum and IL-2. The effect of the combined treatment was greater than either therapies alone.
Similarly in horses with sarcoids (a characteristic equine fibro-epithelial tumor of the skin) the therapeutic effect of cis-platin and local IL-2 was greater than the therapeutic effect of either therapies alone [88].
Indrova et al. [54] studied mice with HPV-16 associated tumors. Peritumoral IL-2 administration could substantially inhibit growth of tumor residua after chemotherapy with cyclophosphamide or ifosfamide derivatives.
Cabanes et al. [20] treated mice bearing M109 pulmonary adenocarcinoma with a combination of liposomal doxorubicin and free IL-2. Both the tumor and the IL-2 application were i.v. or both were i.p. There were synergistic effects with long-term survivors.
Green et al. [48] treated melanoma cases with multiple cutaneous and/or subcutaneous metastases. Metastases were treated with imiquinod daily for 4 weeks before the introduction of intralesional IL-2. This was injected up to three times a week. 182 lesions in a total of 10 patients were treated. A clinical response was seen in 50.5% of the lesions with 40.7% being CR. Furthermore patients with clinically responding cutaneous lesions experienced a marked slowing of the appearance of new lesions. No cutaneous lesions that responded reappeared on cessation of the treatment. Imiquimod alone is often sufficient to elicit a response in purely cutaneous lesions. The addition of intralesional IL-2 however greatly increased the response rate in subcutaneous lesions, and in otherwise refractory cutaneous melanomas.
Conclusion
Combined therapy of locally applied IL-2 and surgery, radiotherapy or chemotherapy may lead to a synergistic therapeutic effect. The durable complete responses of melanoma metastases in the lung after combined chemotherapy and IL-2 suggested that the metastases were eradicated [35].
Mechanism of tumor regression due to local IL-2 application
Original hypothesis on the mechanism of IL-2 in tumor destruction
The original hypothesis about the function of systemically applied IL-2 in cancer therapy was that IL-2 induced Leucocyte Activated Killer cells (LAK cells; [15, 81, 82]. LAK cells were thought to invade the tumor and to kill tumor cells.The following data are incompatible with the cytotoxic LAK-cell hypothesis or any kind of cytotoxic cell hypothesis:
-
Hardly any intravenously injected LAK-cell reaches the tumor [9].
-
In the DBA/2-SL2 lymphoma model it is impossible to induce cytotoxic LAK cells [67].Yet IL-2 therapy is very effective in this model.
-
No general correlation was established between in vitro sensitivity to the cytolytic activity of LAK cells and the antitumor effects observed in vivo [10].
-
Various types of leucocytes may dominate the tumor infiltrate after IL-2 application: macrophages, T cells, NK cells, NKT cells, plasma cells, neutrophilic, eosinophilic cells etc., [70]. The assumption that all these different types of cytotoxic cells are directly induced by IL-2 in different tumors is too complex to be true. In contrast IL-2 should induce a similar mechanism against a wide range of (sensitive) cancers.
-
In addition, when SL2 lymphoma is growing both i.p. and s.c., and if IL-2 is injected i.p. at day 10 after tumor transplantation, then in about 50% of the cases the s.c. tumor regresses completely at about day 17, this in spite of the tumor containing only 0–2% leucocytes [70]. Furthermore, effector/target cell ratios of cytotoxic T-cells are usually very low (in the order of 1:50). It is very unlikely that such a huge tumor is rejected by these few leukocytes. So, cytotoxic cells are not the major cause of tumor regression in this experiment [68].
As this hypothesis was at least at some points wanting to explain the mechanism of the antitumor activity of IL-2, we started to study the histopathology of IL-2 induced tumor regression as study of the histopathology of the IL-2 induced reaction would be essential.
IL-2 induced histopathology in normal tissue [60]
The study of the histopathological events during IL-2-induced tumor regression proved to be extremely difficult. The breakthrough came by serendipity. Professor Hennink and his group at the Faculty of Pharmacy, Utrecht University, are developing slow release systems (microspheres), among others for IL-2. To study the biocompatibility of these microspheres, we injected microspheres loaded with IL-2 subcutaneous in rats [60]. We discovered that the microspheres could be stained with PAS and thus these stained microspheres marked the IL-2 release site. This possibility of a precise localisation of IL-2 was a great tool to further analyse the function of the released IL-2. In a series of sections obtained during the IL-2 induced process we could trace a sequence of reactions. First a localized edema developed, related to swelling of the endothelial lining of the capillaries. After 3 days necrosis of the connective tissue was observed in the center of this edematous area. About a week later there were three zones around this necrotic area. Near the necrotic border was a zone of mixed inflammatory cells. The second zone was a large area of edematous connective tissue. The outer zone showed angiogenesis, a massive proliferation of macrophages around the newly formed blood vessels and also an influx of lymphocytes. About 3–5 week later these macrophages invaded the necrotic material showing features of phagocytosis. Finally also plasma cells and occasionally eosinophils appeared in the peri-necrotic inflammatory area. The plasma cells indicate the induction of an immune reaction.
In short two main phases could be ascertained in this process:
-
an almost immediate marked edema due to leakage of the local blood vessels;
-
a later and more complex reaction consisting of the induction of angiogenesis, a macrophage reaction, migration of the infiltrate into the seminecrotic tissue and finally clearance of tissue in combination with granulomatous processes.
Next we again studied the histopathology of the mechanism of tumor regression induced by free IL-2. Of course the histopathological events after injection of IL-2 in or around a tumor are far more complex than the histopathological events after subcutaneous injection of IL-2 loaded microspheres in normal tissue in rats. But the events after injection of IL-2 at the site of tumors were similar to those after injection of IL-2 loaded microspheres in normal tissue. It is remarkable, however, that IL-2 induced tumor regression may be fast, requiring only some days, or slow, requiring several months, or intermediate. These differences can be related to histopathological differences of the tumor tissues. Before we describe these differences we have to pay attention to some hemodynamic features in tumors.
Some hemodynamic features in tumors
In tumors the interstitial fluid pressure is higher in the center of the tumor mass than in surrounding tissue due to vessel leakage [98]. The increased vascular leakage in tumors is explained as follows:
-
The tumor vessels usually lack a continuous basal membrane [26].
-
Endothelial injury in ischemic tissue [49].
-
Tumors have poor homeostatic control of the circulation due to lack of smooth muscle and lack of innervation [25].
This vascular leakage causes increase of interstitial pressure. This pressure will cause vascular compression, particularly of the post-capillary venules. This leads to stagnation of the blood flow and vascular distension of the prestenotic vessels and sometimes thrombosis in smaller blood vessels. Stagnation of blood causes necrosis, in particular in the tumor center. A peripheral rim of vital tumor tissue often survives as it is just outside the area of deficient blood circulation.
Local IL-2 application into/near the tumor appeared to induce additional edema in and around the tumor, just as in normal tissue. Increased edema exerts extra pressure that causes further stagnation of the blood flow and of the lymph drainage. Obviously, this leads to tumor necrosis and thrombosis within only a few days. This early edema is illustrated with photographs [8, 24, 60, 64].
Fast tumor regression of well-vascularized tumors (Fig. 2) [8]
It is remarkable that IL-2 induced tumor regression is very quick in the case of (very) fast growing tumors like SL2 lymphoma and P815 mastocytoma [67, 68]. Similarly, relatively fast growing cases of mammary carcinoma [73] or BOSCC are more responsive to IL-2 than slower growing BOSCC [91]. In fast growing tumors the intratumoral vascular tree is relatively poorly developed and also the endothelial lining of the microvessels is abnormal. Local IL-2 application will further stimulate vascular leakage. So we assume that much fluid will leak from the tumor vessels, leading to edema, microthrombosis and extensive hemorrhages in the tumor. It is noteworthy that soon after IL-2 injection the tumor becomes firmer, indicating swelling of the tumor mass by edema. This leads to extensive necrosis.
Of course, this large necrotic tumor mass will induce an acute clearance reaction to remove this necrotic debris, which causes an early reduction in size of the tumor mass. This induces a marked immune reaction. It is also noteworthy that this tumor necrosis is accompanied by marked angiogenesis. Adjacent to many of the newly formed smaller blood vessels there is a cuff of proliferating macrophages, which ultimately move into the necrotic debris. As different tumors differ in tumor associated antigen make-up, they will induce also different types of immune reactions, dominated by macrophages, T cells, plasma cells, or eosinophilic cells. This phenomenon can be observed in biopsies taken late at the margin of the disappearing tumor. As a result of the increased tumor immunity isolated tumor strands may be enveloped by granulomatous inflammatory tissue destroying tumor cells. Specific antitumor immunity induced by IL-2 has been described by Maas et al. [67, 68]. After an animal is cured, a second implant of the same tumor is rejected [6, 68, 96].
Our present hypothesis (Fig. 2) seems now most straightforward to explain the fast tumor regression induced by IL-2 in fast growing tumors: The primary function of locally applied IL-2 is the induction of vascular leakage. This leads to an acute massive tumor necrosis and clearance of necrotic tumor material. As a consequence of massive liberation of antigenic tumor material an immune reaction develops. The different types of dominant cytotoxic cells (CTL, eosinophils, macrophages etc.,) depend on the character of the tumor-associated antigens released in the necrotic tumor debris.
According to this view, maximal therapeutic results will be obtained with maximal edema formation within the tumor. In line with this view is the finding that IL-2 is more effective when injected directly into the tumor than when injected peritumorally [56].
It is interesting that it was originally thought that IL-2 killed tumor cells through induction of cytotoxic LAK cells and that the vascular leakage seemed a nasty side effect [7]. According to our present views, it is just the other way round: vascular leakage causing edema is the primary effect of IL-2; the development of systemic immunity [68, 96] seems the secondary effect.
An intriguing point was for many years that in the DBA/2-SL2 model local IL-2 therapy is not effective 1–10 days after tumor cell transplantation, whereas it can be effective after 10 days until about 2 days before the expected death [37, 67]. Similarly, Maekawa et al. [69] found that local IL-2 therapy was only effective when IL-2 therapy was given seven or more days after transplantation of myeloma X5563. This can be explained by the observation that these days are required for developing a corona of angiogenesis around the tumor; this is essential for an effective local IL-2 therapy.
Slow tumor regression of less vascularized tumors
A number of tumors show a slow IL-2 induced regression. Examples are BOSCC [30, 84] and sarcoids in the skin of horses [88]. This regression pattern more closely follows the histological changes as observed in the model, in which the IL-2-releasing microspheres were deposited in normal subcutaneous tissue [60]. Also in these slowly growing tumors edema will be induced in the tumor, but this is more focal at the site of the injection and does not involve the entire tumor. Also the necrotic focus develops slower and the necrosis is less hemorrhagic. In these tumors the complete development of the IL-2 induced response takes at least some weeks. So, the tumor immunity will develop slower and consequently tumor regression will be slower. Moreover in BOSCC the tumor contains only about 50% of tumor cells; the other 50% is stroma. When a the tumor is reduced in size by 50%, then usually hardly any or no tumor cell is present anymore; the remaining 50% is stroma. After killing of the tumor cells, the final regression of the stroma requires several additional months.
Of course both processes (edema and leukocyte infiltration after neoangiogenesis) are always present as a result of local IL-2 application. But in fast-regression models edema is more prevailing, in slow-regression models leukocyte and particularly macrophage infiltration dominate.
Solid subcutaneous SL2 tumors expand by infiltrating the surrounding tissue. These tumor strands are the first targets of macrophages, as the tumor strands are in close proximity to surrounding tumor stroma and are progressively destroyed [8]. So, the body of the tumor and the infiltrating tumor strands are destroyed in two different ways, namely by edema due to vascular leakage and a granulomatous antitumor response originating in the tumor surrounding stroma, respectively.
Obviously IL-2 may stimulate the existing inflammation as often present in tumors and hence attracts more macrophages and inflammatory cells, and as a consequence intensifies the already existing cellular response to the tumor. Usually, there is in the perivascular zones a marked increase of varied types of inflammatory cells.
The reduction of the tumor can be accelerated by using IL-2 in combination with a tumor necrosis inducing agent, as is demonstrated by local cis-platin treatment of mice with MOT tumors [14] and sarcoids in horses [88]. Everse et al. [38] showed that irradiation stimulated the therapeutic effect of IL-2 therapy in mice. We assume that the necrotic material is phagocytosed, thus boosting an immune reaction.
A remarkable finding is that IL-2 injected into the primary tumor can induce regression not only of the primary tumor but also the metastasis in the draining lymph node [6, 96]. However, after surgical removal of the primary tumor IL-2 cannot induce regression of the lymph node metastasis [6]. We assume that this depends on the differences between the histological structure of the primary tumors. Usually in primary tumors there is extensive vascularization and leucocyte infiltration and the tumor is in close contact with the surrounding stroma. In contrast, early tumors in lymphatic tissue often have a scanty vascularization and are situated within the lymph nodes without a direct stroma contact.
Tumor tolerance and local IL-2
In the previous sections histopathological techniques were used to study the mechanism of IL-2 induced tumor regression. In this section we will also pay some attention to immunological data.
Besides stimulating immune responses, IL-2 may also inhibit immune responses. This is clear as deficiency of IL-2 leads to autoimmunity[1]. IL-2 inhibits the immune response by stimulation of CD4+CD25+ T regulatory cells (Treg). These cells suppress immune reactivity including antitumor immune responses [3, 75]. The presence of antigen [77] and IL-2 are important in the maintenance of CD4+CD25+ regulatory T lymphocytes. Depletion of CD4+CD25+ Treg is important for tumor rejection [99]. Depletion of Treg with anti-CD25 antibody stimulates tumor rejection [76].
Because of this tolerance inducing role of IL-2 through Treg, some authors have suggested to use IL-15 for cancer immunotherapy. IL-15 is a cytokine that is functionally closely related to IL-2, but it does not induce tolerance [74]. In 1995, the first animal studies for anti-cancer therapy were performed with IL-15. Although IL-15 therapy still lacks therapeutic successes in human clinic, some authors still suggest that IL-15 should replace IL-2 for immunotherapy of cancer [3].
Importantly and in contrast to IL-15, IL-2 is capable of breaking tolerance [4, 11, 39, 70]. This may be crucial for effective anti-cancer immunotherapy. This reversal of tolerance is mediated through the activation of immature dendritic cells [63]. Activation of intratumoral dendritic cells and reversal of tolerance exerted through local regulatory CD4+CD25+ T lymphocytes, could be the link between local effects of intratumoral IL-2 therapy and systemic immunity. Stimulation of systemic immunity by local IL-2 therapy is also suggested by clinical data on cytokines in treated human patients. Pro-inflammatory cytokines (IFN-gamma, IL-5) are more increased after local IL-2 therapy than anti-inflammatory cytokines. In contrast, anti-inflammatory cytokines (IL-10) are increased after systemic IL-2 therapy [92]. These data highlight mechanistic differences between local and systemic IL-2 therapy.
The discussion above may lead to the question, whether the specific immune system is involved in IL-2 induced tumor rejection. Pleiotropic effects of high local concentrations of IL-2 could lead to aspecific activation of T cells, activation of NK cells and the generation of LAK cells. Nevertheless, the immune system must be involved as local IL-2 treatment causes systemic immunity, that resides in CD3+ lymphocytes or CD4+ and CD8+ lymphocytes [69, 70].
Conclusions
Local IL-2 application seems to induce severe vascular leakage in well-vascularized and fast growing tumors. This leakage in fast growing tumors induces a massive hemorrhagic necrosis which results in early clearance of the tumor tissue. In less-vascular tumor tissue the edema formation is more limited. Neoangiogenesis allows the arrival of especially macrophages that move to the tumor site. This leads to tumor cell killing and a specific immune reaction, resulting in tumor regression. The character of this immune reaction probably depends on the different types of tumor-associated antigens present in the seminecrotic tumor debris. According to our views the primary effects of local IL-2 application to a tumor are the vascular phenomena followed by a host of complex histological events clearing the tumor necrosis. The immune reactivity seems to be a secondary and presumably an indirect effect.
Of course, this is an analysis at the histopathological level. The whole process is far more complicated as immune regulatory events––including cells and cytokines are involved.
Characteristics of local IL-2 therapy
-
Local IL-2 treatment of cancer often cures also the metastases [67, 68, 96]
-
This systemic effect is probably the result of IL-2 induced systemic immunity [67, 68].
-
Local IL-2 therapy can cure large local tumors [31, 67, 68, 88] and even large metastasized tumor burdens [6, 67, 68, 96].
-
Local IL-2 therapy is effective against a large variety of spontaneous carcinomas, melanoma, fibrosarcoma, equine sarcoid, mesothelioma, lung metastases of renal cell carcinoma and melanoma (references see Tables 3, 4). Of course, local IL-2 application is no panacea for all tumors and all types of cancer all the time.
-
Intratumoral IL-2 application is more effective than peritumoral (juxtatumoral) application [55] or application at a more distant site [68].
-
A single IL-2 application may be sufficient [28]. This can be advantageous during surgery when a tumor cannot be excised completely.
-
Local IL-2 therapy is particularly effective in fast growing tumors [67, 68, 73, 91].
-
Most of the tumors that well respond to local IL-2 treatment are those with an already (pre-)existing peritumoral leukocytic infiltrate.
-
Local treatment of the primary tumor may result in regression of both the primary tumor and a metastasis in the draining regional lymph nodes [6, 96].
-
IL-2 induced tumor regression can be fast [24, 67, 68], but it is often a slow process requiring months [30, 31, 88]. The fast tumor regression is often observed in fast growing highly vascularized tumors in which IL-2 causes vascular leakage, leading to edema and intravascular thrombosis followed by necrosis. This is also a consequence of the often abnormal endothelial lining of the intratumoral vascular tree. Slow tumor regression is often observed in poorly vascularized tumors with prominent macrophage infiltration after IL-2 induced neovascularization and an activation of the existing round cell infiltrate. Prior induction of tumor necrosis and local inflammation may accelerate the effect of IL-2.
-
Local IL-2 therapy can exert a synergistic therapeutic effect with surgery, radiotherapy and chemotherapy (references see Table 6). Thus local IL-2 treatment can be a valuable addition to the standard oncotherapy.
-
Local IL-2 has minor side effects and is generally well tolerated (references see Table 5). It can usually be applied without difficulty. It does not require complex technical procedures.
-
Local IL-2 does not hamper or interfere with the standard oncotherapy.
The local IL-2 therapy findings suggest to revisit our views on the relation between local and systemic oncotherapy at least as far as IL-2 therapy concerns
Systemic chemotherapy/hormone therapy, and later immunotherapy, were initially particularly reserved for patients in which adequate tumor treatment by surgery and radiotherapy was not possible. In most of these cases the tumor was inoperable or disseminated and often the patient was in the terminal phase of the disease. Now with modern therapeutic regimes the indications of systemic treatment have been extended, whereas local tumor treatment is becoming rare. Data described in this paper show that cancer treatment with local IL-2 application can lead to cure of metastases. We stress that there are good reasons to consider IL-2 tumor treatment also in early cancer.
Surgical treatment remains the golden standard in malignant tumors. Local IL-2 therapy may be considered in local tumors if these tumors can not be resected completely. Occasionally a previously inoperable lesion might become fit for surgical interventions due to the reduction of the tumor size. In some cases local IL-2 application may be considered as an adjunct to the standard treatment particularly if recurrences are expected. An example of the latter is local IL-2 treatment in TUR-treated bladder tumors.
Local IL-2 therapy may also be used as an adjunct to treat a primary tumor showing metastases. In advanced tumor cases local IL-2 treatment of the primary tumor may induce a specific immune response and might also contribute to cure or decrease tumor size and sometimes also may have a positive effect on the metastases. IL-2 does not interfere with the standard oncotherapy and might be an useful adjunct to diminish suffering and expand lifespan.
Dose at the tumor site Only about 0.1% of the systemically applied dose reaches the tumor. In other words the same dose injected locally results at the site of the tumor in a 1,000 times higher concentration than a systemically applied dose.
Reduced side effects An advantage of local treatment is furthermore that side effects, both local and systemic, are modest if present at all, since the required local dose is only a fraction of the dose required in systemic treatment.
No immunologic overflow feed-back Systemic IL-2 therapy causes immuno suppression as indicated by systemic IL-10 production. This is probably the body’s reaction to a generally activated immune system (cytokine storm). Local IL-2 avoids this down-regulating feedback, and leads to systemic increase of pro-inflammatory cytokines, like IFN-gamma and IL-5.
Reduced costs of locally applied low doses Systemic chemotherapy and immunotherapy in cows and horses is no option. Cattle and horses are so large that systemic oncotherapy is out of order; e.g., required doses of IL-2 would be very large and hence far too expensive in animal husbandry. This problem can be circumvented by local therapy. The results in cattle and horses show that local IL-2 therapy in these large animals is feasible, readily to apply, economic, and therapeutically effective.
Local IL-2 application: doses and duration of the treatment
The number of IL-2 injections required for optimal treatment is still a point of debate. A single injection containing 2 up to 16 million U IL-2 is sufficient in the DBA/2–SL2 model [28], whereas doses of only 5,000 U IL-2 were required in case daily injections on five consecutive days were given [67]. However, Kusnierczyk et al. [64] cured C57BL mice with s.c. solid colon carcinoma with multiple IL-2 treatments, whereas no cure was obtained by a single IL-2 injection. Treating spontaneous BOSCC with a single dose of 2 million U IL-2 [28] gave comparable results as daily IL-2 injection of the same dose on ten consecutive working days [30]. For logistic reasons most clinicians (both in human and in veterinary medicine) strongly prefer to apply one single dose instead of daily injections over five or ten consecutive working days. A single large dose can also be appropriate for tumor treatment during surgery when the tumor mass cannot completely be removed. But such a single dose should be much higher (about 4.5 × 106 IU IL-2) than doses on five or ten consecutive working days (ca 5,000 IU per day in mice).
Very different IL-2 doses, time schedules and routes of application were used. In a human patient we might consider <106 U IL-2 as low dose, (1–18) × 106 U as intermediate dose, and >18 × 106 U IL-2 high dose for local IL-2 application. But a systemically applied dose is far more toxic than the same dose after local application. In addition, the same dose given i.p. in a mouse can be regarded to be high, whereas this dose is low when it is given intravenously in a human patient. A systemically applied dose of 106 U IL-2 is intermediate. But if this dose is given daily for 100 days, then it is a large total dose.
Nowadays we usually treat cancer with a single local injection of 4.5 × 106 U IL-2. This dose is based on data obtained in mice, cattle and dogs [28]. It may be astounding that in local treatment the same dose is effective in a mouse as well as in cattle. In fact similar local processes have to be induced in these different species. In systemic application however the dose required by a cow has to be about 20,000 fold of that used in mice.
We preferably inject IL-2 inside the tumor. This leads to better therapeutic results than peritumoral IL-2 application [56].
Website
Our website on local IL-2 therapy is available at http://cancerimmunotherapy.net/
We provide information targeted on medical professionals. Readers are invited to co-operation. Please reach us at w.denotter@uu.nl.
References
Altman A, Theofilopoulos AN, Weiner R, Katz DH, Dixon FJ (1981) Analysis of T cell function in autoimmune murine strains. Defects in production and responsiveness to interleukin 2. J Exp Med 154:791–808
Anderson PM, Katsanis E, Leonard AS, Schow D, Loeffler CM, Goldstein MB, Ochoa AC (1990) Increased local antitumor effects of interleukin 2 liposomes in mice with MCA-106 sarcoma pulmonary metastases. Cancer Res 50:1853–1856
Antoni PA, Restifo NP (2005) CD4+CD25+ T regulatory cells, immunotherapy of cancer, and interleukin-2. J Immunother 28:120–128
Appleman LJ, Boussiotis VA (2003) T cell anergy and costimulation. Immunol Rev 162:161–180
Astoul P, Picat-Joossen D, Viallat JR, Boutin C (1999) Intrapleural administration of interleukin-2 for the treatment of patients with malignant pleural mesothelioma: a Phase II study. Cancer 86:546–547
Balemans LT, Steerenberg PA, Koppenhagen FJ, Kremer BH, De Mulder PHM, Claessen AM, Scheper RJ, Den Otter W (1994) PEG-IL-2 therapy of advanced cancer in the guinea pig. Impact of the primary tumor and beneficial effect of cyclophosphamide. Int J Cancer 58:871–876
Baluna R, Vitetta E (1997) Vascular leak syndrome: a side effect of immunotherapy. Immunopharmacology 37:117–132
Baselmans AHC, Koten JW, Battermann JJ, Van Dijk JE, Den Otter W (2002) The mechanism of regression of solid SL2 lymphosarcoma after local IL-2 therapy. Cancer Immunol Immunother 51:492–498
Basse PH (1995) Tissue distribution and tumor localisation of effector cells in adoptive immunotherapy of cancer. APMIS Suppl 55:1–28
Belardelli F, Ciolli V, Testa U, Montesoro E, Bulgarini D, Proietti E, Borghi P, Sestili P, Locardi P, Peschle C, et al. (1989) Anti-tumor effect of interleukin-2 and interleukin-1 in mice transplanted with different syngeneic tumors. Int J Cancer 44:1108–1116
Bendiksen S, Rekvig OP (2004) Interleukin-2, but not interleukin-15, is required to terminate experimentally induced clonal T-cell anergy. Scand J Immunol 60:64–73
Bernsen MR, Dullens HFJ, Den Otter W, Heinz APM (1995) Re-evaluation of the superiority of polyethylene glycol modified Interleukin-2 over regular recombinant IL-2. J Interferon and Cytokine Res 15:641–645
Bernsen MR, Tang J-W, Everse LA, Koten JW, Den Otter W (1999) Interleukin-2 (IL-2) therapy: potential advantages of locoregional versus systemic administration. Cancer Treatment Rev 25:73–82
Bernsen MR, Van der Velden AW, Everse LA, Dullens HFJ, Den Otter W, Heinz APM (1998) Interleukin-2: Hope in cases of Cisplatin resistant tumours. Cancer Immunol Immunother 6:41–47
Bubenik J, Indrova M (1987) The anti-tumour efficacy of human recombinant interleukin 2. Correlation between sensitivity of tumours to the cytolytic effect of LAK cells in vitro and their susceptibility to interleukin 2 immunotherapy in vivo. Cancer Immunol Immunother 24:269–271
Bubenik J, Indrova M, Perlmann P, Berzins K, Mach O, Kraml J, Toulcova A (1985) Tumour-inhibitory effects of TCGF (IL-2)-containing preparations. Cancer Immunol Immunother 9:57–61
Bubenik J, Kieler J, Indrova M (1986) Local treatment with human recombinant IL-2 inhibits growth of MC-induced sarcomas in syngeneic mice. Folia Biol (Praha) 32:209–211
Bubenik J, Perlmann P, Indrova M, Simova J, Jandlova T, Neuwirt J (1983) Growth inhibition of an MC-induced mouse sarcoma by TCGF (IL-2) containing preparations. Cancer Immunol Immunother 14:205–206
Burdin ML (1964) Squamous-cell carcinoma of the vulva in cattle in Kenia. Res Vet Sci 4:497–505
Cabanes A, Even-Chen S, Zimberoff J, Barenholz Y, Kedar E, Gabizon A (1999) Enhancement of antitumor activity of polyethylene glycol-coated liposomal doxorubicin with soluble and liposomal interleukin-2. Clin Cancer Res 5:687–693
Casana PH, Hernandez H, Arana MJ (2002) Interleukin-2 inhibits proliferation of HPV-associated tumor cells and halts tumor growth in vivo. Biochem Biophys Res Commun 299:818–824
Castagneto B, Zai S, Mutti L, Lazzaro A, Ridolfi R, Piccolini F, Ardizzoni A, Fumagalli L, Valsuani G, Botta M (2001) Palliative and therapeutic activity of IL-2 immunotherapy in unresectable malignant pleural mesothelioma with pleural effusion: results of a phase II study on 31 consecutive patients. Lung Cancer 31:303–310
Chi KH, Myers JN, Chow KC, Chan WK, Tsang YW, Chao Y, Yen SH, Lotze MT (2001) Phase II trial of systemic recombinant interleukin-2 in the treatment of refractory nasopharyngeal carcinoma. Oncology 60:110–115
De Mik HJI, Koten JW, Maas RA, Dullens HFJ, Den Otter W (1991) Tumour regression by IL-2 mediated stagnation of blood flow. In Vivo 5:679–684
Denekamp J (1984) Vascular endothelium as vulnerable element in tumours. Acta Radiol Oncol 23:217–225
Den Otter W et al (1998) Letter. J Urol 160:1808
Den Otter W, Balemans L, Battermann JJ, 17 other authors (1999) Local low-dose IL-2 therapy. Hepatogastroenterology 46(suppl.1):1280–1286
Den Otter W, Cadée J, Gavhumende R, De Groot CJ, Hennink WE, Stewart R (1999) Effective cancer therapy with a single injection of interleukin-2 at the site of the tumour. Cancer Immunol Immunother 48:419–420
Den Otter W, Dobrowolski Z, Bugajski A, Papla B, Van der Meijden APM, Koten JW, Boon TA, Siedlar M, Zembala M (1998) Intravesical IL-2 to treat T1 papillary carcinoma : regression of marker lesions in 8 out of 10 patients. J Urol 159:1183–1186
Den Otter W, Hill FWG, Klein WR, Koten JW, Steerenberg PA, De Mulder PHM, Rhode C, Stewart R, Faber JAJ, Rutten VPMG (1995) Therapy of bovine ocular squamous cell carcinoma with local doses of Interleukin-2 : 67% complete regressions after 20 months of follow-up. Cancer Immunol Immunother 41:10–14
Den Otter W, Hill FWG, Klein WR, Koten JW, Steerenberg PA, De Mulder PHM, Rutten VPMG, Ruitenberg EJ (1993) Low doses of interleukin-2 can cure large bovine ocular squamous cell carcinoma. Anticancer Res 13:2453–2455
Den Otter W, Steerenberg PA, Van der Laan JW (2002) Testing therapeutic potency of anticancer drugs in animal studies: a commentary. Regul Toxicol Pharmacol 35:266–272
Dubinett SM, Patrone L, Tobias J, Cochran AJ, Wen DR, McBride WH (1993) Intratumoral interleukin-2 immunotherapy: activation of tumor-infiltrating and splenic lymphocytes in vivo. Cancer Immunol Immunother 36:156–162
Edwards RP, Gooding W, Lembersky MC, Colonello K, Hammond R, Paradise C, Kowal CD, Kunschner AJ, Baldisser IM, Kirkwood JM, Herberman RB (1997) Comparison of toxicity and survival following intraperitoneal recombinant Interleukin-2 for persistent ovarian cancer after platinum: twenty-four-hour versus 7-day infusion. J Clin Oncol 15:3399–3407
Enk AH, Nashan D, Rubben A, Knop J (2000) High dose inhalation interleukin 2 therapy for lung metastases in patients with malignant melanoma. Cancer 2042–2046
Esteban-González E, Carballido J, Navas V, Torregrosa Z, Munoz A, de Mon MA, PortugueseSpanish Inhaled IL-2 Group (2007) Prospective review in patients with pulmonary metastases of renal cell carcinoma receiving inhaled recombinant interleukin-2. Anticancer Drugs 18:291–296
Everse LA, Bernsen MR, Dullens HFJ, Den Otter W (1996) The success of locoregional low-dose recombinant interleukin-2 (rIL-2) therapy in tumor-bearing mice is dependent on the time of rIL-2 administration. J Exp Ther Oncol 1:231–236
Everse LA, Renes B, Jürgenliemk-Schulz IM, Rutgers DH, Bernsen MR, Dullens HFJ, Den Otter W, Battermann JJ (1997) Local low-dose interleukin-2 induces systemic immunity when combined with radiotherapy of cancer. A pre-clinical study. Int J Cancer 72:1003–1007
Fasler S, Aversa G, Terr A, Thestrup-Pedersen K, de Vries JE, Yssel H (1995) Peptide-induced anergy in allergen-specific human Th2 cells results in lack of cytokine production and B cell help for IgE synthesis. Reversal by IL-2, not by IL-4 or IL-13. J Immunol 155:4199–4206
Ferlazzo G, Magno C, Iemmo R, Rizzo M, Lupo G, Semino C, Bruno S, Melioli G (1996) Treatment of superficial bladder cancer with intravesical perfusion of rIL-2: a follow-up study. Anticancer Res 16:979–980
Ferlazzo G, Magno C, Lupo G, Rizzo M, Iemmo R, Semino C, Melioli G (1995) A phase I study of intravesical continuous perfusion of recombinant interleukin-2 in patients with superficial bladder cancer. Am J Clin Oncol 16:100–104
Fiszer-Maliszewska L, Den Otter W, Mordarski M (1999) Effect of local interleukin-2 treatment on spontaneous tumours of different immunogenic strength. Cancer Immunol Immunother 47:306–314
Goey SH, Eggermont AM, Punt CJ, Slingerland R, Gratama JW, Oosterom R, Oskam R, Bolhuis RL, Stoter G (1995) Intrapleural administration of interleukin-2 in pleural mesothelioma: a phase I-II study. Br J Cancer 72:1283–1288
Gomella LG, McGinnis DE (1998) Letter to the Editor. Urology 160:1808
Gomella LG, McGinnis DE, Baltish MA, Thompson I, Butler K, Marshall E (1993) Treatment of superficial transitional cell carcinoma of the bladder with recombinant interleukin-2 (rIL-2) (CETUS). Cancer Biother 8:223–227
Grande C, Firvida JL, Navas V, Casal J (2006) Interleukin-2 for the treatment of solid tumors other than melanoma and renal cell carcinoma. Anticancer Drugs 17:1–12
Grasso M, Torelli F, Scannapieco G, Franzoso F, Lania C (2001) Neoadiuvant treatment with intravesical Interleukin-2 for recurrent superficial transitional bladder carcinoma Ta-T1/G1–2. J Immunother 24:184–187
Green DS, Bodman-Smith MD, Dalgleish AG, Fischer MD (2007) Phase I/II study of topical imiquinod and intralesional interleukin-2 in the treatment of accessible metastases in malignant melanoma. Br J Dermatol 156:337–345
Gregoriadis G, Poste G (1984) Drug targeting in cancer therapy. In: Senior J, Trouet A (eds), Receptor-mediated targeting of drugs. Plenum Press, New York, pp 448–451
Hautmann SH, Huland E, Huland H (1999) Local intratumor immunotherapy of prostate cancer with interleukin-2 reduces tumor growth. Anticancer Res 19:2661–2663
Hill FWG, Klein WR, Hoyer HJ, Rutten VPMG, Kock N, Steerenberg PA, Ruitenberg EJ, Den Otter W (1994) Antitumor effect of locally injected low doses of recombinant human interleukin-2 in bovine vulval papilloma and carcinoma. Vet Immunol Immunopathol 41:19–29
Huland E, Heinzer H, Mir TS, Huland H (1997) Inhaled interleukin-2 therapy in pulmonary metastatic renal cell carcinoma. Cancer J Sci Am 3(Suppl 1):S98-S105
Huland E, Huland H (1989) Local continuous high dose interleukin 2: a new therapeutic model for the treatment of advanced bladder carcinoma. Cancer Res 49:5469–5474
Indrova M, Bubenik J, Mikyskova R, Mendoza L, Simova J, Bieblova J, Jandlova T, Jinoch P, Smahel M, Vonka V, Pajtasz-Piasecka E (2003) Chemoimmunotherapy in mice carrying HPV16-associated, MHC class I+ and class I- tumours: Effects of CBM-4A potentiated with IL-2, IL-12, GM-CSF and genetically modified tumour vaccines. Int J Oncol 22:691–695
Jacobs JJL, Hordijk GJ, Jürgenliemk-Schulz IM, Terhaard CHJ, Koten JW, Battermann JJ, Den Otter W (2005) Treatment of stage III-IV nasopharyngeal carcinomas by external beam irradiation and local low doses of IL-2. Cancer Immunol Immunother 54:792–798
Jacobs JJL, Sparendam D, Den Otter W (2005) Local interleukin-2 therapy is therapeutically more effective against cancer when injected intratumourally. Cancer Immunol Immunother 54:647–654
Janssen RA, Mulder NH, The TH, de Leij L (1994) The immunobiological effects of interleukin-2 in vivo. Review. Cancer Immunol Immunother 39:207–216
Jürgenliemk-Schulz IM, Renes IB, Rutgers DH, Everse LA, Bernsen MR, Den Otter W, Battermann JJ (1997) Anti-tumor effects of local irradiation in combination with peritumoral administration of low doses of recombinant interleukin-2 (rIL-2). Radiat Oncol Investig 5:54–61
Kaplan B, Moy RL (2000) Effect of perilesional injections of PEG-interleukin-2 on basal cell carcinoma. Dermatol Surg 26:1037–1040
Koten JW, Van Luyn MJA, Cadée JA, Brouwer L, Hennink WE, Bijleveld C, Den Otter W (2003) IL-2 loaded dextran microspheres with attractive histocompatibility properties for local IL-2 cancer therapy. Cytokine 24:57–66
Krastev Z, Koltchakov C, Tomova R, Deredjian S, Alexiev A, Popov D, Tomov B, Koten JW, Jacobs J, Den Otter W (2005) Locoregional IL-2 low dose application for gastrointestinal tumors. World J Gastroenterol 11:5525–5529
Krastev Z, Koltchakov V, Vladov N, Popov D, Milev A, Koten JW, Den Otter W (2001) A mesothelioma that is sensitive to locally applied IL-2. Cancer Immunol Immunother 50:226–227
Kubo T, Hatton RD, Oliver J, Liu X, Elson CO, Weaver CT (2004) Regulatory T cell suppression and anergy are differentially regulated by proinflammatory cytokines produced by TLR-activated dendritic cells. J Immunol 173:7249–7258
Kusnierczyk H, Pajtasz-Piasecka E, Koten JW, Bijleveld C, Krawczyk K, Den Otter W (2004) Further development of local IL-2 therapy of cancer: multiple versus single IL-2 treatment of transplanted murine colon carcinoma. Cancer Immunol Immunother 53:445–452
Lissoni P, Barni S, Ardizzoia A, Paolorossi F, Tisi E, Crispino S, Tancini G (1992) Intracavitary administration of interleukin-2 as palliative therapy for neoplastic effusions. Tumori 78:118–120
Liu CM, Suzuki Y, Chen LP, Okayasu T, Calkins CE, Wheelock EF (1990) Maintenance and cure of the L5178Y murine tumor-dormant state by interleukin-2: in vivo and in vitro effects. Cancer Res 50:1361–1367
Maas RA, Dullens HFJ, De Jong WH, Den Otter W (1989) Immunotherapy of mice with a large burden of disseminated lymphoma with low-dose interleukin-2. Cancer Res 49:7037–7040
Maas RA, Van Weering DHJ, Dullens HFJ, Den Otter W (1991) Intratumoral low-dose interleukin-2 induces rejection of distant solid tumour. Cancer Immunol Immunother 33:389–394
Maekawa R, Matsumoto M, Kitagawa T, Harada M, Sato K (1986) Effect of recombinant IL-2 (R-IL-2) on in vivo growth of murine myeloma X5563. Cancer Immunol Immunother 23:25–30
Margolin KA (2000) Interleukin-2 in the treatment of renal cancer. Semin Oncol 27:194–203
Masotti A, Fumagalli L, Morandini GC (1997) Intrapleural administration of recombinant interleukin-2 in non-small cell lung cancer with neoplastic pleural effusion. Monaldi Arch Chest Dis 52:225 228
Mattijssen V, Balemans LTM, Steerenberg PA, De Mulder PHM (1992) Polyethylene-glycol-modified interleukin-2 is superior to interleukin-2 in locoregional immunotherapy of established guinea pig tumours. Int J Cancer 51:812–817
Moiseeva EV, Merkulova IB, Bijleveld C, Koten JW, Miroshnikov AI, Den Otter W (2003) Therapeutic effect of a single dose of IL-2 on transplanted murine breast cancer. Cancer Immunol Immunother 52:487–496
Munger W, Dejoy SQ, Wick MM, Kerwar SS (1995) Studies on evaluating the antitimour acticity and toxicity of interleukin-15, a new T cell growth factor: comparison with interleukin-2. Cell Immunol 165:289–293
Nelson BH (2004) IL-2 regulatory T cells, and tolerance. J Immunol 172:3983–3988
Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E (1999) Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res 59:3129–3133
Onodera K, Volk HD, Ritter T, Kupiec-Weglinski JW (1998) Thymus requirement and antigen dependency in the “Infectious” tolerance pathway in transplant recipients. J Immunol 160:5765–5772
Pfohler C, Steinhauser S, Wagner A, Ugurel S, Tilgen W (2004) Complete remission of cutaneous satellite and in-transit metastases. After intralesional therapy with interleukin-2 in 2 patients with malignant melanoma Hautarzt. 55:171–175 (Article in German)
Pizza G, Severini G, Menniti D, De Vinci C, Corrado F (1984) Tumour regression after intralesional injection of interleukin-2 (IL-2) in bladder cancer. Preliminary report. Int J Cancer 34:359–367
Radny P, Caroli UM, Bauer J, Paul T, Schlegel C, Eigentler TK, Weide B, Schwartz M, Garbe C (2003) Phase II trial of intralesional therapy with interleukin-2 in soft tissue melanoma metastases. Br J Cancer 89:1620–1626
Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE, Matory YL, Skibber JM, Shiloni E, Vetto JT, Seipp CA, Simpson C, Reichert CM (1985) Observations on the systemic administration of autologous lymphokine activated killer cells and recombinant IL-2 to patients with metastatic cancer. New Engl J Med 313:1485–1492
Rosenberg SA, Mulé, Spiess PJ, Reichert CM, Schwarz SL (1985) Regression of established pulmonary metastases and subcutaneous tumor mediated by the systemic administration of high-dose recombinant interleukin-2. J Exp Med 161:1169–1188
Rosenstein M, Ettinghausen SE, Rosenberg SA (1986) Extravasation of intravascular fluid mediated by the systemic administration of recombinant interleukin 2. J Immunol 137:1735–1742
Rutten VPMG, Klein WR, De Jong WAC, Misdorp W, DenOtter W, Steerenberg PA, De Jong WH, Ruitenberg EJ (1989) Local IL-2 therapy in bovine ocular squamous cell carcinoma. A pilot study. Cancer Immunol Immunother 30:165–169
Shirai M, Watanabe S, Nishioka M (1990) Antitumour effect of intratumoral injection of human recombinant interleukin-2 in patients with hepatocellular carcinoma: a preliminary report. Eur J Cancer 26:1045–1048
Silagi S, Schaefer AE (1986) Successful immunotherapy of mouse melanoma and sarcoma with recombinant interleukin-2 and cyclophosphamide. J Biol Resp Mod 5:411–422
Sosnovski JT, DeHaven JI, Riggs DR, Lamm DL (1991) Treatment of murine transitional cell carcinoma with intralesional interleukin-2 and murine interferon gamma. J Urol 146:1164–1167
Spoormakers TJP, Klein WR, Jacobs JJL, Van den Ingh ThSGAM, Koten JW, Den Otter W (2003) Comparison of the local treatment of equine sarcoids with IL-2 or cisplatin/IL-2. Cancer Immunol Immunother 52:179–184
Stewart RJE, Hill FWG, Masztalerz A, Jacobs JJL, Koten JW, Den Otter W (2006) Treatment of ocular squamous cell carcinomas in cattle using interleukin-2. Vet Rec 159:668–672
Stewart RJE, Masztalerz A, Jacobs JJL, Den Otter W (2005) Local interleukin-2 and interleukin-12 therapy of bovine ocular squamous cell carcinomas. Vet Immunol Immunopathol 106:277–284
Taylor DD, Edwards RP, Case CR, Gercel-Taylor C (2004) Modulation of CD3-zeta as a marker of clinical response to IL-2 therapy in ovarian cancer patients. Gynecol Oncol 94:54–60
Tomova R, Pomakov J, Jacobs JJL, Adjarov D, Popova S, Altankova I, Den Otter W, Krastev Z (2006) Changes in cytokine profile during local IL-2 therapy in cancer patients. Anticancer Res 26:2037–2047
Tubaro A, Stoppacciaro A, Velotti F, Bossola PC, Cusumano G, Vicentini C, De Carli P, Ruco L, Santoni A, Cancrini A, et al. (1995) Local immunotherapy of superficial bladder cancer by intravesical instillation of recombinant interleukin 2. Eur Urol 28:297–303
Vaage J (1987) Local and systemic effects during interleukin-2 therapy of mouse mammary tumors. Cancer Res 47:4296–4298
Vaage J (1991) Peri-tumor interleukin-2 causes systemic therapeutic effect via interferon-gamma induction. Int J Cancer 49:598–600
Van Es R, Baselmans AHC, Koten JW, Van Dijk JE, Koole R, Den Otter W (2000) Perilesional IL-2 treatment of a VX2 head and neck cancer model can induce a systemic anti-tumour activity. Anticancer Res 20:4163–4170
Vlock DR, Snyderman CH, Johnson JT, Myers EN, Eibling DE, Rubin JS, Kirkwood JM, Dutcher JP, Adams GL (1994) Phase Ib trial of the effect of peritumoral and intranodal injections of interleukin-2 in patients with advanced squamous cell carcinoma of the head and neck: an Eastern Cooperative Oncology Group trial. J Imunother Emphasis Tumor Immunol 15:134–139
Wiig H (1982) Microvascular pressures in DMBA-induced rat mammary tumors. Scand J Clin Lab Invest 42:165–171
Yu P, Lee Y, Liu W, Krausz T, Chong A, Schreiber H, Fu YX (2005) Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J Exp Med 201:779–791
Ziekman PGPM (1999) Local low-dose IL-2 therapy of tumours in companion animals. Anticancer Res 19:1995
Acknowledgments
These studies were supported by the K.F. Hein Fonds, Utrecht and by Chiron/Novartis. The cartoon was drawn by Anne-Marie Keegstra.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Den Otter, W., Jacobs, J.J.L., Battermann, J.J. et al. Local therapy of cancer with free IL-2. Cancer Immunol Immunother 57, 931–950 (2008). https://doi.org/10.1007/s00262-008-0455-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-008-0455-z