Abstract
Purpose
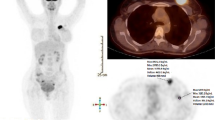
To assess the feasibility and first experience of combined 18F-FDG-PET)/dynamic contrast-enhanced (DCE) CT in evaluating breast cancer.
Methods
Nine consecutive female patients (mean age 64.2 years, range 52–74 years) with primary breast carcinoma were prospectively recruited for combined 18F-FDG PET/DCE-CT. Dynamic CT data were used to calculate a range of parameters of tumour vascularity, and tumour 18F-FDG uptake (standardized uptake value, SUVmax) was used as a metabolic indicator.
Results
One tumour did not enhance and was excluded. The mean tumour SUVmax was 7.7 (range 2.4–26.1). The mean values for tumour perfusion, perfusion normalized to cardiac output, standard perfusion value (SPV) and permeability were 41 ml/min per 100 g (19–59 ml/min per 100 g), 0.56%/100 g (0.33–1.09%/100 g), 3.6 (2.5–5.9) and 0.15/min (0.09–0.30/min), respectively. Linear regression analysis showed a positive correlation between tumour SUV and tumour perfusion normalized to cardiac output (r=0.55, p=0.045) and a marginal correlation between tumour SUV and tumour SPV (r=0.19, p=0.065). There were no significant correlations between tumour SUV and tumour perfusion (r=0.29, p=0.401) or permeability (r=0.03, p=0.682).
Conclusion
The first data from combined 18F-FDG-PET/DCE-CT in breast cancer are reported. The technique was successful in eight of nine patients. Breast tumour metabolic and vascular parameters were consistent with previous data from 15O-H2O-PET.


Similar content being viewed by others
References
Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006;24:2137–50.
Fletcher JW, Djulbegovic B, Soares HP, Siegel BA, Lowe VJ, Lyman GH, et al. Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med 2008;49:480–508.
Tatsumi M, Cohade C, Mourtzikos KA, Fishman EK, Wahl RL. Initial experience with FDG-PET/CT in the evaluation of breast cancer. Eur J Nucl Med Mol Imaging 2006;33:254–62.
Syed R, Bomanji JB, Nagabhushan N, Hughes S, Kayani I, Groves A, et al. Impact of combined (18)F-FDG PET/CT in head and neck tumours. Br J Cancer 2005;92:1046–50.
Lardinois D, Weder W, Hany TF, Kamel EM, Korom S, Seifert B, et al. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med 2003;348:2500–7.
Bacharach SL, Libutti SK, Carrasquillo JA. Measuring tumor blood flow with H(2)(15)O: practical considerations. Nucl Med Biol 2000;27:671–6.
Miles KA, Griffiths MR, Keith CR. Blood flow-metabolic relationships are dependent on tumour size in non-small cell lung cancer: a study using quantitative contrast-enhanced computer tomography and positron emission tomography. Eur J Nucl Med Mol Imaging 2006;33:22–8.
Hayes C, Padhani AR, Leach MO. Assessing changes in tumour vascular function using dynamic contrast-enhanced magnetic resonance imaging. NMR Biomed 2002;15:154–63.
Mankoff DA, Dunnwald LK, Gralow JR, Ellis GK, Schubert EK, Tseng J, et al. Changes in blood flow and metabolism in locally advanced breast cancer treated with neoadjuvant chemotherapy. J Nucl Med 2003;44:1806–14.
Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999. EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer 1999;35:1773–82.
Smith IC, Welch AE, Hutcheon AW, Miller ID, Payne S, Chilcott F, et al. Positron emission tomography using [18F]-fluorodeoxy-D-glucose to predict the pathologic response of breast cancer to primary chemotherapy. J Clin Oncol 2000;18:1676–88.
Semple SI, Gilbert FJ, Redpath TW, Staff RT, Ahearn TS, Welch AE. The relationship between vascular and metabolic characteristics of primary breast tumours. Eur Radiol 2004;14:2038–45.
Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991;19:403–10.
Miles KA. Measurement of tissue perfusion by dynamic computed tomography. Br J Radiol 1991;64:409–12.
Miles KA, Griffiths MR, Fuentes MA. Standardised perfusion value: a universal contrast enhancement scale that correlates with FDG-PET in lung cancer. Radiology 2001;220:548–53.
Miles KA, Young H, Chica SL, Esser PD. Quantitative contrast-enhanced computed tomography: is there a need for system calibration? Eur Radiol 2007;17:919–26.
Miles KA, Kelley BB. CT measurements of capillary permeability within nodal masses: a potential technique for assessing the activity of lymphoma. Br J Radiol 1997;70:74–9.
Mankoff D, Dunnwald LK, Gralow JR, Georgiana EK, Charlop A, Lawton TJ, et al. Blood flow and metabolism in locally advanced breast cancer: relationship to response to therapy. J Nucl Med 2002;43:500–9.
Anderson H, Price P. Clinical measurement of blood flow in tumours using positron emission tomography: a review. Nucl Med Commun 2002;23:131–8.
Hentschel M, Paulus T, Mix M, Moser E, Nitzsche EU, Brink I. Analysis of blood flow and glucose metabolism in mammary carcinomas and normal breast: a H2(15)O PET and 18F-FDG PET study. Nucl Med Commun 2007;28:789–97.
Hirasawa H, Tsushima Y, Hirasawa S, Takei H, Taketomi-Takahasi A, Takano A, et al. Perfusion CT of breast carcinoma: arterial perfusion of nonscirrhous carcinoma was higher than that of scirrhous carcinoma. Acad Radiol 2007;14:547–52.
Liu Y, Bellomi M, Gatti G, Ping X. Accuracy of computed tomography perfusion in assessing metastatic involvement of enlarged axillary lymph nodes in patients with breast cancer. Breast Cancer Res 2007;9:R40.
Brix G, Henze M, Knopp MV, Lucht R, Doll J, Junkermann H, et al. Comparison of pharmacokinetic MRI and [18F]fluorodeoxyglucose PET in the diagnosis of breast cancer: initial experience. Eur Radiol 2001;11:2058–70.
Groheux D, Moretti JL, Baillet G, Espie M, Giacchetti S, Hindie E, et al. Effect of (18)F-FDG PET/CT imaging in patients with clinical Stage II and III breast cancer. Int J Radiat Oncol Biol Phys 2008;71:695–704.
Acknowledgments
The Breast Cancer Campaign (UK charity) funded this work. This work was undertaken at UCLH UCL, which received a proportion of the funding from the NIHR Biomedical Research Centres funding scheme of the UK Department of Health. Gordon Wishart receives some funding from the Cambridge Biomedical Research Centre. None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this article. We acknowledge the expertise of Professor Brian Hutton, Chair of Nuclear Medicine Physics, for his advice on normalizing SUV. We are grateful to Alison Hallett and other staff of the Cambridge Breast Unit for their help with this project, and thank the patients for volunteering for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Groves, A.M., Wishart, G.C., Shastry, M. et al. Metabolic–flow relationships in primary breast cancer: feasibility of combined PET/dynamic contrast-enhanced CT. Eur J Nucl Med Mol Imaging 36, 416–421 (2009). https://doi.org/10.1007/s00259-008-0948-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-008-0948-1