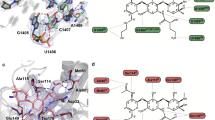
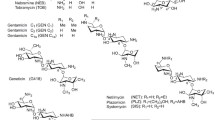
Abstract
Aminoglycosides are potent bactericidal antibiotics targeting the bacterial ribosome, where they bind to the A-site and disrupt protein synthesis. They are particularly active against aerobic, Gram-negative bacteria and act synergistically against certain Gram-positive organisms. Aminoglycosides are used in the treatment of severe infections of the abdomen and urinary tract, bacteremia, and endocarditis. They are also used for prophylaxis, especially against endocarditis. Bacterial resistance to aminoglycosides continues to escalate and is widely recognized as a serious health threat. This might be the reason for the interest in understanding the mechanisms of resistance. It is now clear that the resistance occurs by different mechanisms such as prevention of drug entry, active extrusion of drugs, alteration of the drug target (mutational modification of 16S rRNA and mutational modification of ribosomal proteins), and enzymatic inactivation through the expression of enzymes, which covalently modify these antibiotics. Enzymatic inactivation is normally due to acetyltransferases, nucleotidyltransferases, and phosphotransferases. In this review, we focus on the recent concept of molecular understanding of aminoglycoside action and resistance.



Similar content being viewed by others
References
Ahmed AM, Shimamoto T (2004) A plasmid-encoded class 1 integron carrying sat, a putative phosphoserine phosphatase gene and aadA2 from enterotoxigenic Escherichia coli O159 isolated in Japan. FEMS Microbiol Lett 235:243–248
Aires JR, Kohler T, Nikaido H, Plesiat P (1999) Involvement of an active efflux system in the natural resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob Agents Chemother 43:2624–2628
Azucena E, Mobashery S (2001) Aminoglycoside-modifying enzymes: mechanisms of catalytic processes and inhibition. Drug Resist Updat 4:106–117
Ban N, Nissen P, Hansen J, Moore PB, Steitz TA (2000) The complete atomic structure of the large ribosomal subunit at 2.4 Å resolution. Science 289:905–920
Boehr DD, Daigle D, Wright GD (2004) Domain–domain interactions in the aminoglycoside antibiotic resistance enzyme AAC(6’)-APH(2"). Biochemistry 43:9846–9855
Boehr DD, Farley AR, LaRonde FJ, Murdock TR, Wright GD, Cox JR (2005) Establishing the principles of recognition in the adenine-binding region of an aminoglycoside antibiotic kinase [APH(3’)-IIIa]. Biochemistry 44:12445–12553
Bryan LE, Kwan S (1983) Roles of ribosomal binding, membrane potential, and electron transport in bacterial uptake of streptomycin and gentamicin. Antimicrob Agents Chemother 23:835–845
Burk DL, Hon WC, Leung AK, Berghuis AM (2001) Structural analyses of nucleotide binding to an aminoglycoside phosphotransferase. Biochemistry 40:8756–8764
Burk DL, Ghuman N, Wybenga-Groot L-E, Berghuis AM (2004) X-ray structure of the AAC(6’)-Ii antibiotic resistance enzyme at 1.8 A resolution; examination of oligomeric arrangements in GNAT superfamily members. Protein Sci 12:426–437
Cate JH, Yusupov MM, Yusupova GZ, Earnest TE, Noller HF (1999) X-ray crystal structure of 70S ribosome functional complexes. Science 285:2095–2104
Cho J, Hamasaki K, Rando RR (1998) The binding site of a specific aminoglycoside binding RNA molecule. Biochemistry 37:4985–4992
Davies J, Wright GD (1997a) Bacterial resistance to aminoglycoside antibiotics. Trends Microbiol 5:63–70
Davies J, Wright GD (1997b) Bacterial resistance to aminoglycoside antibiotics. Trends Microbiol 5:234–240
Doi Y, Yokoyama K, Yamane K, Wachino J, Shibata N, Yagi T, Shibayama K, Kato H, Arakawa Y (2004) Plasmid-mediated 16S rRNA methylase in Serratia marcescens conferring high-level resistance to aminoglycosides. Antimicrob Agents Chemother 48:491–496
Draker KA, Wright GD (2004) Molecular mechanism of the enterococcal aminoglycoside 6’-N-acetyltransferase’: role of GNAT-conserved residues in the chemistry of antibiotic inactivation. Biochemistry 43:446–454
Dworkin RJ (1999) Aminoglycosides for the treatment of gram-negative infections: therapeutic use, resistance and future outlook. Drug Resist Updat 2:173–179
Fluit AC, Schmitz FJ (1999) Class 1 integrons, gene cassettes, mobility, and epidemiology. Eur J Clin Microbiol Infect Dis 18:761–770
Fourmy D, Recht MI, Blanchard SC, Puglisi JD (1996) Structure of the A-site of E. coli. 16 S rRNA complexed with an aminoglycoside antibiotic. Science 274:1367–1371
Fourmy D, Yoshizawa S, Puglisi JD (1998) Paromomycin binding induces a local conformational change in the A-site of 16 S rRNA. J Mol Biol 277:333–345
Galimand M, Courvalin P, Lambert T (2003) Plasmid-mediated high-level resistance to aminoglycosides in Enterobacteriaceae due to 16S rRNA methylation. Antimicrob Agents Chemother 47:2565–2571
Galimand M, Sabtcheva S, Courvalin P, Lambert T (2005) Worldwide disseminated armA aminoglycoside resistance methylase gene is borne by composite transposon Tn1548. Antimicrob Agents Chemother 49:2949–2953
Gates CA, Northrop DB (1988) Substrate specificities and structure-activity relationships for the nucleotidylation of antibiotics catalyzed by aminoglycoside nucleotidyltransferase 2"-I. Biochemistry 27:3820–3825
Giamerllou H (1986) Aminoglycosides plus beta-lactams against Gram-negative organisms. Am J Med 80:126–137
Hancock RE, Farmer SW, Li ZS, Poole K (1991) Interaction of aminoglycosides with the outer membranes and purified lipopolysaccharide and OmpF porin of E. coli. Antimicrob Agents Chemother 35:1309–1314
Hatch RA, Schiller NL (1998) Alginate lyase promotes diffusion of aminoglycosides through the extracellular polysaccharide of mucoid Pseudomonas aeruginosa. Antimicrob Agents Chemother 42:974–977
Hayashi SF, Norcia LJ, Seibel SB, Silvia AM (1997) Structure-activity relationships of hygromycin A and its analogs: protein synthesis inhibition activity in a cell free system. J Antibiot (Tokyo) 50:514–521
Hegde SS, Javid-Majd F, Blanchard JS (2001) Overexpression and mechanistic analysis of chromosomally encoded aminoglycoside 2’-Nacetyltransferase (AAC(2’)-Ic) from Mycobacterium tuberculosis. J Biol Chem 276:45876–45881
Hon WC, McKay GA, Thompson PR, Sweet RM, Yang DS, Wright GD, Berghuis AM (1997) Structure of an enzyme required for aminoglycoside antibiotic resistance reveals homology to eukaryotic protein kinases. Cell 89:887–895
Hotta K, Zhu CB, Ogata T, Sunada A, Ishikawa J, Mizuno S, Kondo S (1996) Enzymatic 2’-N-acetylation of arbekacin and antibiotic activity of its product. J Antibiot (Tokyo) 49:458–464
Jana S, Deb JK (2005) Molecular targets for design of novel inhibitors to circumvent aminoglycoside resistance. Curr Drug Targets 6(3):353–361
Jana S, Karan G, Deb JK (2005) Purification of Streptomycin adenylyltransferase from a recombinant Escherichia coli. Protein Expr Purif 40:86–90
Jiang L, Patel DJ (1998) Solution structure of the tobramycin-RNA aptamer complex. Nat Struct Biol 5:769–774
Kotra LP, Haddad J, Mobashery S (2000) Aminoglycoside: perspectives on mechanisms of action and resistance and strategies to counter resistance. Antimicrob Agents Chemother 44:3249–3256
Levings RS, Partridge SR, Lightfoot D, Hall RM, Djordjevic SP (2005) New integron-associated gene cassette encoding a 3-N-aminoglycoside acetyltransferase. Antimicrob Agents Chemother 49:1238–1241
Livermore DM (2002) Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin Infect Dis 34:634–640
Llano-Sotelo B, Azucena EF, Kotra LP, Mobashery S, Chow CS (2002) Aminoglycosides modified by resistance enzymes display diminished binding to the bacterial ribosomal aminoacyl-tRNA site. Chem Biol 9:455–463
Magnet S, Blanchard JS (2005) Molecular insights into aminoglycoside action and resistance. Chem Rev 105:477–497
Maravic G (2004) Macrolide resistance based on the Erm-mediated rRNA methylation. Curr Drug Targets Infect Disord 4:193–202
Masuda N, Sakagawa E, Ohya S, Gotoh N, Tsujimoto H, Nishino T (2000) Substrate specificities of MexAB-OprM, MexCD-OprJ, and MexXY-OprM efflux pumps in Pseudomonas aeruginosa. Antimicrob Agents Chemother 44:3322–3327
McKay GA, Wright GD (1995) Kinetic mechanism of aminoglycoside phosphotransferase type IIIa: evidence for a Theorell–Chance mechanism. J Biol Chem 270:24686–24692
McKay GA, Roestamadji J, Mobashery S, Wright GD (1996) Recognition of aminoglycoside antibiotics by the enterococcal/staphylococcal aminoglycoside 3’-phosphotransferase type IIIa: role of substrate amino groups. Antimicrob Agents Chemother 40:2648–2650
Meier A, Kirschner P, Bange F-C, Vogel U, Bottger E-C (1994) Genetic alterations in streptomycin-resistant Mycobacterium tuberculosis: mapping of mutations conferring resistance. Antimicrob Agents Chemother 38:228–233
Melancon P, Tapprich WE, Brakier-gingras L (1992) Single-base mutations at positions 2661 of E. coli. 23 S rRNA increase efficiency of translational proofreading. J Bacteriol 174:7896–7901
Michael K, Wang H, Tor Y (1999) Enhanced RNA binding of dimerized aminoglycosides. Bioorg Med Chem 7:1361–1371
Mingeot-Leclercq MP, Glupczynski Y, Tulkens PM (1999) Aminoglycosides: activity and resistance. Antimicrob Agents Chemother 43:727–737
Moazed D, Noller NF (1987) Interaction of antibiotics with functional sites in 16S ribosomal RNA. Nature 327:389–394
Neu HC (1992) The crisis in antibiotic resistance. Science 257:1064–1073
Noller HF (1991) Ribosomal RNA and translation. Annu Rev Biochem 60:191–227
Nurizzo D, Shewry SC, Perlin MH, Brown SA, Dholakia JN, Fuchs RL, Deva T, Baker EN, Smith CA (2004) The crystal structure of aminoglycoside-3’-phosphotransferase-IIa, an enzyme responsible for antibiotic resistance. J Mol Biol 327:491–506
Pedersen LC, Benning MM, Holden HM (1995) Structural investigation of the antibiotic and ATP-binding sites in kanamycin nucleotidyl transferases. Biochemistry 34:13305–13311
Peloquin CA, Berning SE, Nitta AT, Simone PM, Goble M, Huitt GA, Iseman MD, Cook JL, Curran-Everett D (2004) Aminoglycoside toxicity: daily versus thrice-weekly dosing for treatment of mycobacterial diseases. Clin Infect Dis 38:1538–1544
Pezzella C, Ricci A, DiGiannatale E, Luzzi I, Carattoli A (2004) Tetracycline and streptomycin resistance genes, transposons, and plasmids in Salmonella enterica isolates from animals in Italy. Antimicrob Agents Chemother 48:903–908
Pilch DS, Kaul M, Barbieri CM (2005) Defining the basis for the specificity of aminoglycoside–rRNA recognition: a comparative study of drug binding to the A sites of Escherichia coli and human rRNA. J Mol Biol 346:119–134
Poole K (2005) Efflux-mediated antimicrobial resistance. J Antimicrob Chemother 56:20–51
Puglisi JD, Fourmy D, Recht ML (1998) Binding of neomycin-class aminoglycoside antibiotics to the A-site of 16 S rRNA. J Mol Biol 277:347–362
Recht MI, Fourmy D, Blanchard SC, Dahlquist KD, Puglisi JD (1996) RNA sequence determinants for aminoglycoside binding to an A-site rRNA model oligonucleotide. J Mol Biol 262:421–436
Ristuccia AM, Cunha BA (1982) The aminoglycosides. Drug Discov Today 66:303–312
Roestamadji J, Grapsas I, Mobashery S (1995) Loss of individual electrostatic interactions between aminoglycoside antibiotics and resistance enzymes as an effective means to overcoming bacterial drug resistance. J Am Chem Soc 117:11060–11069
Rougier F, Claude D, Maurin M, Maire P (2004) Aminoglycoside nephrotoxicity. Curr Drug Targets 4:153–162
Rybak LP, Whitworth CA (2005) Ototoxicity: therapeutic opportunities. Drug Discov Today 10:1313–1321
Sakon J, Liao HH, Kanikula AM, Benning MM, Rayment I, Holden HM (1993) Molecular structure of kanamycin nucleotidyl transferase determined to 3 Å resolution. Biochemistry 32:11977–11984
Shaw KJ, Rather PN, Hare RS, Miller GH (1993) Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol Rev 57:138–163
Spelman DW, McDonald M, Spice WJ (1989) Aminoglycoside antibiotic agents: a review. Therapeutics 151:346–349
Sunada A, Nakajima M, Ikeda Y, Kondo S, Hotta K (1999) Enzymatic 1-N-acetylation of paromomycin by an actinomycete strain # 8 with multiple aminoglycoside resistance and paromomycin sensitivity. J Antibiot (Tokyo) 52:809–814
Vakulenko SB, Mobashery S (2003) Versatility of aminoglycosides and prospects for their future. Clin Microbiol Rev 16(3)430–450
Vetting MW, Magnet S, Nieves E, Roderick SL, Blanchard JS (2004) A bacterial acetyltransferase capable of regioselective N-acetylation of antibiotics and histones. Chem Biol 433:212–226
Vetting MW, Sde Carvalho LP, Yu M, Hegde SS, Magnet S, Roderick SL, Blanchard JS (2005) Structure and functions of the GNAT superfamily of acetyltransferases. Arch Biochem Biophys 433:212–226
Walmsley M (2001) The structure and function of drug pumps. Trends Microbiol 9:71–79
Walsh C (2000) Molecular mechanisms that confer antibacterial drug resistance. Nature 406:775–781
Watanabe A, Nagai J, Adachi Y, Katsube T, Kitahara Y, Murakami T, Takanz M (2004) Targeted prevention of renal accumulation and toxicity of gentamicin by aminoglycoside binding receptor antagonists. J Control Release 95:423–433
Welch KT, Virga KG, Whittemore NA, Ozen C, Wright E, Brown CL, Lee RE, Serpersu EH (2005) Discovery of non-carbohydrate inhibitors of aminoglycoside-modifying enzymes. Bioorg Med Chem 13:6252–6363
Westbrock-Wadman S, Sherman D-R, Hickey M-J, Coulter SN, Zhu YQ, Warrener P, Nguyen LY, Shawar RM, Folger KR, Stover CK (1999) Characterization of a Pseudomonas aeruginosa efflux pump contributing to aminoglycoside impermeability. Antimicrob Agents Chemother 43:2975–2983
Wright GD (2005) Bacterial resistance to antibiotics: enzymatic degradation and modification. Adv Drug Deliv Rev 57:1451–1470
Wright E, Serpersu EH (2004) Isolation of aminoglycoside nucleotidyltransferase(2")-Ia from inclusion bodies as active, monomeric enzyme. Protein Expr Purif 35:373–380
Wybenga-Groot LE, Draker K, Wright GD, Berghuis AM (1999) Crystal structure of an aminoglycoside 6’-N-acetyltransferase: defining the GCN5-related N-acetyltransferase superfamily fold. Structure Fold Des 7:497–507
Xi H, Arya DP (2005) Recognition of triple helical nucleic acids by aminoglycosides. Curr Med Chem Anti Canc Agents 5:327–338
Yamane K, Wachino J, Doi Y, Kurokawa H, Arakawa Y (2005) Global spread of multiple aminoglycoside resistance genes. Emerg Infect Dis 11:951–953
Yao S, Sgarbi PW, Marby KA, Rabuka D, O’Hare SM, Cheng ML, Bairi M, Hu C, Hwang S-B, Hwang C-K (2004) Glyco-optimization of aminoglycosides: new aminoglycosides as novel anti-infective agents. Bioorg Med Chem Lett 14:3733–3738
Zembower TR, Noskin GA, Postelnick MJ, Nguyen C, Peterson LR (1998) The utility of aminoglycosides in an era of emerging drug resistance. Int J Antimicrob Agents 10:95–105
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jana, S., Deb, J.K. Molecular understanding of aminoglycoside action and resistance. Appl Microbiol Biotechnol 70, 140–150 (2006). https://doi.org/10.1007/s00253-005-0279-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-005-0279-0