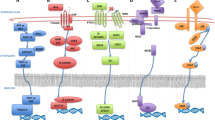
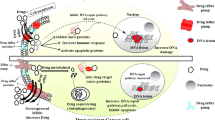
Abstract
Chemotherapy is one of the most effective and broadly used approaches for cancer management and many modern regimes can eliminate the bulk of the cancer cells. However, recurrence and metastasis still remain a major obstacle leading to the failure of systemic cancer treatments. Therefore, to improve the long-term eradication of cancer, the cellular and molecular pathways that provide targets which play crucial roles in drug resistance should be identified and characterised. Multidrug resistance (MDR) and the existence of tumor-initiating cells, also referred to as cancer stem cells (CSCs), are two major contributors to the failure of chemotherapy. MDR describes cancer cells that become resistant to structurally and functionally unrelated anti-cancer agents. CSCs are a small population of cells within cancer cells with the capacity of self-renewal, tumor metastasis, and cell differentiation. CSCs are also believed to be associated with chemoresistance. Thus, MDR and CSCs are the greatest challenges for cancer chemotherapy. A significant effort has been made to identify agents that specifically target MDR cells and CSCs. Consequently, some agents derived from nature have been developed with a view that they may overcome MDR and/or target CSCs. In this review, natural products-targeting MDR cancer cells and CSCs are summarized and clustered by their targets in different signaling pathways.

Similar content being viewed by others
References
Gilbertson RJ (2011) Mapping cancer origins. Cell 145(1):25–29. doi:10.1016/j.cell.2011.03.019
O’Connor R, Clynes M, Dowling P, O’Donovan N, O’Driscoll L (2007) Drug resistance in cancer—searching for mechanisms, markers and therapeutic agents. Expert Opin Drug Metab Toxicol 3(6):805–817. doi:10.1517/17425255.3.6.805
Gottesman MM, Fojo T, Bates SE (2002) Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer 2(1):48–58. doi:10.1038/nrc706
Ramachandra M, Ambudkar SV, Chen D, Hrycyna CA, Dey S, Gottesman MM, Pastan I (1998) Human P-glycoprotein exhibits reduced affinity for substrates during a catalytic transition state. Biochemistry 37(14):5010–5019. doi:10.1021/bi973045u
Tsuruo T, Iida H, Tsukagoshi S, Sakurai Y (1981) Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res 41(5):1967–1972
Thimmaiah KN, Horton JK, Qian XD, Beck WT, Houghton JA, Houghton PJ (1990) Structural determinants of phenoxazine type compounds required to modulate the accumulation of vinblastine and vincristine in multidrug-resistant cell lines. Cancer Commun 2(7):249–259
Belinsky MG, Chen ZS, Shchaveleva I, Zeng H, Kruh GD (2002) Characterization of the drug resistance and transport properties of multidrug resistance protein 6 (MRP6, ABCC6). Cancer Res 62(21):6172–6177
McCormack E, Bruserud O, Gjertsen BT (2005) Animal models of acute myelogenous leukaemia—development, application and future perspectives. Leukemia 19(5):687–706. doi:10.1038/sj.leu.2403670
Yu Z, Pestell TG, Lisanti MP, Pestell RG (2012) Cancer stem cells. Int J Biochem Cell Biol 44(12):2144–2151. doi:10.1016/j.biocel.2012.08.022
Vaiopoulos AG, Kostakis ID, Koutsilieris M, Papavassiliou AG (2012) Colorectal cancer stem cells. Stem Cells 30(3):363–371. doi:10.1002/stem.1031
Velasco-Velazquez MA, Homsi N, De La Fuente M, Pestell RG (2012) Breast cancer stem cells. Int J Biochem Cell Biol 44(4):573–577. doi:10.1016/j.biocel.2011.12.020
Tu SM, Lin SH (2012) Prostate cancer stem cells. Clin Genitourin Cancer 10(2):69–76. doi:10.1016/j.clgc.2012.01.002
Ricci-Vitiani L, Fabrizi E, Palio E, De Maria R (2009) Colon cancer stem cells. J Mol Med 87(11):1097–1104. doi:10.1007/s00109-009-0518-4
Piccirillo SG, Binda E, Fiocco R, Vescovi AL, Shah K (2009) Brain cancer stem cells. J Mol Med 87(11):1087–1095. doi:10.1007/s00109-009-0535-3
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB (2004) Identification of human brain tumour initiating cells. Nature 432(7015):396–401. doi:10.1038/nature03128
O’Brien CA, Pollett A, Gallinger S, Dick JE (2007) A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 445(7123):106–110. doi:10.1038/nature05372
Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL (2000) Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA 97(26):14720–14725. doi:10.1073/pnas.97.26.14720
Oshima Y, Suzuki A, Kawashimo K, Ishikawa M, Ohkohchi N, Taniguchi H (2007) Isolation of mouse pancreatic ductal progenitor cells expressing CD133 and c-Met by flow cytometric cell sorting. Gastroenterology 132(2):720–732. doi:10.1053/j.gastro.2006.11.027
Dean M, Hamon Y, Chimini G (2001) The human ATP-binding cassette (ABC) transporter superfamily. J Lipid Res 42(7):1007–1017
Beaulieu E, Demeule M, Ghitescu L, Beliveau R (1997) P-glycoprotein is strongly expressed in the luminal membranes of the endothelium of blood vessels in the brain. Biochem J 326(Pt 2):539–544
Ruetz S, Gros P (1994) Phosphatidylcholine translocase: a physiological role for the mdr2 gene. Cell 77(7):1071–1081
Melaine N, Lienard MO, Dorval I, Le Goascogne C, Lejeune H, Jegou B (2002) Multidrug resistance genes and p-glycoprotein in the testis of the rat, mouse, Guinea pig, and human. Biol Reprod 67(6):1699–1707
Yague E, Raguz S (2010) Escape from stress granule sequestration: another way to drug resistance? Biochem Soc Trans 38(6):1537–1542. doi:10.1042/BST0381537
Smith R, Rathod RJ, Rajkumar S, Kennedy D (2014) Nervous translation, do you get the message? A review of mRNPs, mRNA-protein interactions and translational control within cells of the nervous system. Cell Mol Life Sci 71(20):3917–3937. doi:10.1007/s00018-014-1660-x
Shtil AA, Azare J (2005) Redundancy of biological regulation as the basis of emergence of multidrug resistance. Int Rev Cytol 246:1–29. doi:10.1016/S0074-7696(05)46001-5
Sodani K, Patel A, Kathawala RJ, Chen ZS (2012) Multidrug resistance associated proteins in multidrug resistance. Chin J Cancer 31(2):58–72. doi:10.5732/cjc.011.10329
Scheffer GL, Pijnenborg AC, Smit EF, Muller M, Postma DS, Timens W, van der Valk P, de Vries EG, Scheper RJ (2002) Multidrug resistance related molecules in human and murine lung. J Clin Pathol 55(5):332–339
Peng KC, Cluzeaud F, Bens M, Duong Van Huyen JP, Wioland MA, Lacave R, Vandewalle A (1999) Tissue and cell distribution of the multidrug resistance-associated protein (MRP) in mouse intestine and kidney. J Histochem Cytochem 47(6):757–768
St-Pierre MV, Serrano MA, Macias RI, Dubs U, Hoechli M, Lauper U, Meier PJ, Marin JJ (2000) Expression of members of the multidrug resistance protein family in human term placenta. Am J Physiol Regul Integr Comp Physiol 279(4):R1495–R1503
Jorajuria S, Dereuddre-Bosquet N, Becher F, Martin S, Porcheray F, Garrigues A, Mabondzo A, Benech H, Grassi J, Orlowski S, Dormont D, Clayette P (2004) ATP binding cassette multidrug transporters limit the anti-HIV activity of zidovudine and indinavir in infected human macrophages. Antivir Ther 9(4):519–528
Gibson NM, Quinn CJ, Pfannenstiel KB, Hydock DS, Hayward R (2014) Effects of age on multidrug resistance protein expression and doxorubicin accumulation in cardiac and skeletal muscle. Xenobiot Fate Foreign Compd Biol Syst 44(5):472–479. doi:10.3109/00498254.2013.846489
Wijnholds J, Scheffer GL, van der Valk M, van der Valk P, Beijnen JH, Scheper RJ, Borst P (1998) Multidrug resistance protein 1 protects the oropharyngeal mucosal layer and the testicular tubules against drug-induced damage. J Exp Med 188(5):797–808
Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD (1998) A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA 95(26):15665–15670
Dean M, Rzhetsky A, Allikmets R (2001) The human ATP-binding cassette (ABC) transporter superfamily. Genome Res 11(7):1156–1166. doi:10.1101/gr.184901
Doyle L, Ross DD (2003) Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene 22(47):7340–7358. doi:10.1038/sj.onc.1206938
Summer R, Kotton DN, Sun X, Ma B, Fitzsimmons K, Fine A (2003) Side population cells and Bcrp1 expression in lung. Am J Physiol Lung Cell Mol Physiol 285(1):L97–L104. doi:10.1152/ajplung.00009.2003
Alvi AJ, Clayton H, Joshi C, Enver T, Ashworth A, Vivanco M, Dale TC, Smalley MJ (2003) Functional and molecular characterisation of mammary side population cells. Breast Cancer Res 5(1):R1–R8
Robey RW, Polgar O, Deeken J, To KW, Bates SE (2007) ABCG2: determining its relevance in clinical drug resistance. Cancer Metastas Rev 26(1):39–57. doi:10.1007/s10555-007-9042-6
An G, Gallegos J, Morris ME (2011) The bioflavonoid kaempferol is an Abcg2 substrate and inhibits Abcg2-mediated quercetin efflux. Drug Metab Dispos Biol Fate Chem 39(3):426–432. doi:10.1124/dmd.110.035212
Rabindran SK, He H, Singh M, Brown E, Collins KI, Annable T, Greenberger LM (1998) Reversal of a novel multidrug resistance mechanism in human colon carcinoma cells by fumitremorgin C. Cancer Res 58(24):5850–5858
Allen JD, van Loevezijn A, Lakhai JM, van der Valk M, van Tellingen O, Reid G, Schellens JH, Koomen GJ, Schinkel AH (2002) Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther 1(6):417–425
Nusslein-Volhard C, Wieschaus E (1980) Mutations affecting segment number and polarity in Drosophila. Nature 287(5785):795–801
Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP (1993) Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 75(7):1417–1430
Kawamura S, Hervold K, Ramirez-Weber FA, Kornberg TB (2008) Two patched protein subtypes and a conserved domain of group I proteins that regulates turnover. J Biol Chem 283(45):30964–30969. doi:10.1074/jbc.M806242200
Fiorini L, Tribalat MA, Sauvard L, Cazareth J, Lalli E, Broutin I, Thomas OP, Mus-Veteau I (2015) Natural paniceins from mediterranean sponge inhibit the multidrug resistance activity of Patched and increase chemotherapy efficiency on melanoma cells. Oncotarget 6(26):22282–22297. doi:10.18632/oncotarget.4162
Scales SJ, de Sauvage FJ (2009) Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends Pharmacol Sci 30(6):303–312. doi:10.1016/j.tips.2009.03.007
Bidet M, Tomico A, Martin P, Guizouarn H, Mollat P, Mus-Veteau I (2012) The hedgehog receptor patched functions in multidrug transport and chemotherapy resistance. Mol Cancer Res 10(11):1496–1508. doi:10.1158/1541-7786.mcr-11-0578
Yu JS, Liu GT, Morris-Irvin D, Black KL (2005) Glioblastoma cancer stem cells exhibit chemoresistance with overexpression of multidrug resistance gene BCRP-1. Neurosurgery 57(2):428
Chaudhary PM, Roninson IB (1991) Expression and activity of P-glycoprotein, a multidrug efflux pump, in human hematopoietic stem cells. Cell 66(1):85–94
Venugopal A, Kwatra D, Stecklein S, Ramalingam S, Subramaniam D, Anant S (2012) RNA binding protein RBM3 promotes a cancer stem cell phenotype with multidrug resistance. FASEB J:26
Grimm M, Krimmel M, Polligkeit J, Alexander D, Munz A, Kluba S, Keutel C, Hoffmann J, Reinert S, Hoefert S (2012) ABCB5 expression and cancer stem cell hypothesis in oral squamous cell carcinoma. Eur J Cancer 48(17):3186–3197. doi:10.1016/j.ejca.2012.05.027
Fuchs D, Daniel V, Sadeghi M, Opelz G, Naujokat C (2010) Salinomycin overcomes ABC transporter-mediated multidrug and apoptosis resistance in human leukemia stem cell-like KG-1a cells. Biochem Biophys Res Commun 394(4):1098–1104. doi:10.1016/j.bbrc.2010.03.138
Xu K, Liang X, Cui D, Wu Y, Shi W, Liu J (2013) miR-1915 inhibits Bcl-2 to modulate multidrug resistance by increasing drug-sensitivity in human colorectal carcinoma cells. Mol Carcinog 52(1):70–78. doi:10.1002/mc.21832
Signore M, Ricci-Vitiani L, De Maria R (2013) Targeting apoptosis pathways in cancer stem cells. Cancer Lett 332(2):374–382. doi:10.1016/j.canlet.2011.01.013
Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL, Yu JS (2006) Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer 5:67. doi:10.1186/1476-4598-5-67
Aggarwal BB (2004) Nuclear factor-κB: the enemy within. Cancer Cell 6(3):203–208. doi:10.1016/j.ccr.2004.09.003
Aggarwal BB, Vijayalekshmi RV, Sung B (2009) Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res 15(2):425–430. doi:10.1158/1078-0432.CCR-08-0149
Griffin JD (2001) Leukemia stem cells and constitutive activation of NF-kappaB. Blood 98(8):2291
Baron F, Turhan AG, Giron-Michel J, Azzarone B, Bentires-Alj M, Bours V, Bourhis JH, Chouaib S, Caignard A (2002) Leukemic target susceptibility to natural killer cytotoxicity: relationship with BCR-ABL expression. Blood 99(6):2107–2113
Palayoor ST, Youmell MY, Calderwood SK, Coleman CN, Price BD (1999) Constitutive activation of IkappaB kinase alpha and NF-kappaB in prostate cancer cells is inhibited by ibuprofen. Oncogene 18(51):7389–7394. doi:10.1038/sj.onc.1203160
Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ Jr, Sledge GW Jr (1997) Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Mol Cell Biol 17(7):3629–3639
Aggarwal BB, Sung B (2011) NF-kappaB in cancer: a matter of life and death. Cancer discovery 1(6):469–471. doi:10.1158/2159-8290.CD-11-0260
Zhou J, Zhang H, Gu P, Bai J, Margolick JB, Zhang Y (2008) NF-kappaB pathway inhibitors preferentially inhibit breast cancer stem-like cells. Breast Cancer Res Treat 111(3):419–427. doi:10.1007/s10549-007-9798-y
Stahl M, Ge C, Shi S, Pestell RG, Stanley P (2006) Notch1-induced transformation of RKE-1 cells requires up-regulation of cyclin D1. Cancer Res 66(15):7562–7570. doi:10.1158/0008-5472.CAN-06-0974
Osipo C, Patel P, Rizzo P, Clementz AG, Hao L, Golde TE, Miele L (2008) ErbB-2 inhibition activates Notch-1 and sensitizes breast cancer cells to a gamma-secretase inhibitor. Oncogene 27(37):5019–5032. doi:10.1038/onc.2008.149
Malanchi I, Peinado H, Kassen D, Hussenet T, Metzger D, Chambon P, Huber M, Hohl D, Cano A, Birchmeier W, Huelsken J (2008) Cutaneous cancer stem cell maintenance is dependent on beta-catenin signalling. Nature 452(7187):650–653. doi:10.1038/nature06835
Vermeulen L, De Sousa EMF, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, Sprick MR, Kemper K, Richel DJ, Stassi G, Medema JP (2010) Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol 12(5):468–476. doi:10.1038/ncb2048
Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL (2003) A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 423(6938):409–414. doi:10.1038/nature01593
Wend P, Holland JD, Ziebold U, Birchmeier W (2010) Wnt signaling in stem and cancer stem cells. Semin Cell Dev Biol 21(8):855–863. doi:10.1016/j.semcdb.2010.09.004
Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A (2007) HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol 17(2):165–172. doi:10.1016/j.cub.2006.11.033
Bar EE, Chaudhry A, Lin A, Fan X, Schreck K, Matsui W, Piccirillo S, Vescovi AL, DiMeco F, Olivi A, Eberhart CG (2007) Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells 25(10):2524–2533. doi:10.1634/stemcells.2007-0166
Zahreddine HA, Culjkovic-Kraljacic B, Assouline S, Gendron P, Romeo AA, Morris SJ, Cormack G, Jaquith JB, Cerchietti L, Cocolakis E, Amri A, Bergeron J, Leber B, Becker MW, Pei S, Jordan CT, Wilson HM Jr, Katherine LBB (2014) The sonic hedgehog factor GLI1 imparts drug resistance through inducible glucuronidation. Nature 511(7507):90
Sims-Mourtada J, Izzo JG, Ajani J, Chao KSC (2007) Sonic Hedgehog promotes multiple drug resistance by regulation of drug transport. Oncogene 26(38):5674–5679. doi:10.1038/sj.onc.121035
Linn DE, Yang X, Sun F, Xie Y, Chen H, Jiang R, Chen H, Chumsri S, Burger AM, Qiu Y (2010) A role for OCT4 in tumor initiation of drug-resistant prostate cancer cells. Genes Cancer 1(9):908–916. doi:10.1177/1947601910388271
Wang XQ, Ongkeko WM, Chen L, Yang ZF, Lu P, Chen KK, Lopez JP, Poon RTP, Fan ST (2010) Octamer 4 (Oct4) mediates chemotherapeutic drug resistance in liver cancer cells through a potential Oct4–AKT–ATP-binding cassette G2 pathway. Hepatology 52(2):528–539. doi:10.1002/hep.23692
Landen CN Jr, Goodman B, Katre AA, Steg AD, Nick AM, Stone RL, Miller LD, Mejia PV, Jennings NB, Gershenson DM, Bast RC Jr, Coleman RL, Lopez-Berestein G, Sood AK (2010) Targeting aldehyde dehydrogenase cancer stem cells in ovarian cancer. Mol Cancer Ther 9(12):3186–3199. doi:10.1158/1535-7163.MCT-10-0563
Lingala S, Cui Y-Y, Chen X, Ruebner BH, Qian X-F, Zern MA, Wu J (2010) Immunohistochemical staining of cancer stem cell markers in hepatocellular carcinoma. Exp Mol Pathol 89(1):27–35. doi:10.1016/j.yexmp.2010.05.005
Clay MR, Tabor M, Owen JH, Carey TE, Bradford CR, Wolf GT, Wicha MS, Prince ME (2010) Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck 32(9):1195–1201. doi:10.1002/hed.21315
Tanei T, Morimoto K, Shimazu K, Kim SJ, Tanji Y, Taguchi T, Tamaki Y, Noguchi S (2009) Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin Cancer Res 15(12):4234–4241. doi:10.1158/1078-0432.CCR-08-1479
Lugli A, Iezzi G, Hostettler I, Muraro MG, Mele V, Tornillo L, Carafa V, Spagnoli G, Terracciano L, Zlobec I (2010) Prognostic impact of the expression of putative cancer stem cell markers CD133, CD166, CD44s, EpCAM, and ALDH1 in colorectal cancer. Br J Cancer 103(3):382–390. doi:10.1038/sj.bjc.6605762
Su Y, Qiu Q, Zhang X, Jiang Z, Leng Q, Liu Z, Stass SA, Jiang F (2010) Aldehyde dehydrogenase 1 A1-positive cell population is enriched in tumor-initiating cells and associated with progression of bladder cancer. Cancer Epidemiol Biomark Prev 19(2):327–337. doi:10.1158/1055-9965.EPI-09-0865
Li ZJ, Xiang Y, Xiang LX, Xiao YN, Li FJ, Hao P (2014) ALDH maintains the stemness of lung adenoma stem cells by suppressing the Notch/CDK2/CCNE pathway. PLoS One 9(3):e92669. doi:10.1371/journal.pone.0092669
Kim R-J, Park J-R, Roh K-J, Choi AR, Kim S-R, Kim P-H, Yu JH, Lee JW, Ahn S-H, Gong G, Hwang J-W, Kang K-S, Kong G, Sheen YY, Nam J-S (2013) High aldehyde dehydrogenase activity enhances stem cell features in breast cancer cells by activating hypoxia-inducible factor-2α. Cancer Lett 333(1):18–31. doi:10.1016/j.canlet.2012.11.026
Vasiliou V, Nebert DW (2005) Analysis and update of the human aldehyde dehydrogenase (ALDH) gene family. Hum Genom 2(2):138–143
Cortes-Dericks L, Froment L, Boesch R, Schmid RA, Karoubi G (2014) Cisplatin-resistant cells in malignant pleural mesothelioma cell lines show ALDHhighCD44+ phenotype and sphere-forming capacity. BMC Cancer 14(1):304. doi:10.1186/1471-2407-14-304
Liu J, Xiao ZJ, Wong SKM, Tin VPC, Ho KY, Wang JW, Sham MH, Wong MP (2013) Lung cancer tumorigenicity and drug resistance are enhanced through ALDH(hi)CD44(hi) tumor initiating cells. Oncotarget 4(10):1686–1699
Croker AK, Allan AL (2012) Inhibition of aldehyde dehydrogenase (ALDH) activity reduces chemotherapy and radiation resistance of stem-like ALDHhiCD44+ human breast cancer cells. Breast Cancer Res Treat 133(1):75–87. doi:10.1007/s10549-011-1692-y
Liu P, Brown S, Goktug T, Channathodiyil P, Kannappan V, Hugnot JP, Guichet PO, Bian X, Armesilla AL, Darling JL, Wang W (2012) Cytotoxic effect of disulfiram/copper on human glioblastoma cell lines and ALDH-positive cancer-stem-like cells. Br J Cancer 107(9):1488–1497. doi:10.1038/bjc.2012.442
Zhi QM, Chen XH, Ji J, Zhang JN, Li JF, Cai Q, Liu BY, Gu QL, Zhu ZG, Yu YY (2011) Salinomycin can effectively kill ALDHhigh stem-like cells on gastric cancer. Biomed Pharmacother 65(7):509–515. doi:10.1016/j.biopha.2011.06.006
Maugeri-Saccà M, Bartucci M, De Maria R (2012) DNA damage repair pathways in cancer stem cells. Mol Cancer Ther 11(8):1627–1636. doi:10.1158/1535-7163.MCT-11-1040
Burke BA, Carroll M (2010) BCR-ABL: a multi-faceted promoter of DNA mutation in chronic myelogeneous leukemia. Leukemia 24(6):1105–1112. doi:10.1038/leu.2010.67
Zhang M, Behbod F, Atkinson RL, Landis MD, Kittrell F, Edwards D, Medina D, Tsimelzon A, Hilsenbeck S, Green JE, Michalowska AM, Rosen JM (2008) Identification of tumor-initiating cells in a p53-null mouse model of breast cancer. Cancer Res 68(12):4674–4682. doi:10.1158/0008-5472.CAN-07-6353
Bao S, Dewhirst MW, Hjelmeland AB, Bigner DD, Wu Q, Hao Y, Rich JN, McLendon RE, Shi Q (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444(7120):756–760. doi:10.1038/nature05236
Yan J, Tang DM (2014) Prostate cancer stem-like cells proliferate slowly and resist etoposide-induced cytotoxicity via enhancing DNA damage response. Exp Cell Res 328(1):132–142. doi:10.1016/j.yexcr.2014.08.016
Saito Y, Najima Y, Takagi S, Uchida N, Wake A, Taniguchi S, Sone A, Ishikawa F, Tanaka S, Tomizawa-Murasawa M, Aoki Y, Suzuki N, Shultz LD (2010) Induction of cell cycle entry eliminates human leukemia stem cells in a mouse model of AML. Nat Biotechnol 28(3):275–280. doi:10.1038/nbt.1607
Chen Y, Li D, Wang D, Liu X, Yin N, Song Y, Lu SH, Ju Z, Zhan Q (2012) Quiescence and attenuated DNA damage response promote survival of esophageal cancer stem cells. J Cell Biochem 113(12):3643–3652. doi:10.1002/jcb.24228
Chan JY, Chu AC, Fung KP (2000) Inhibition of P-glycoprotein expression and reversal of drug resistance of human hepatoma HepG2 cells by multidrug resistance gene (mdr1) antisense RNA. Life Sci 67(17):2117–2124
Pan L, Liu J, He Q, Wang L, Shi J (2013) Overcoming multidrug resistance of cancer cells by direct intranuclear drug delivery using TAT-conjugated mesoporous silica nanoparticles. Biomaterials 34(11):2719–2730. doi:10.1016/j.biomaterials.2012.12.040
Ford JM (1995) Modulators of multidrug resistance. Preclinical studies. Hematol Oncol Clin North Am 9(2):337–361
Cornwell MM, Safa AR, Felsted RL, Gottesman MM, Pastan I (1986) Membrane vesicles from multidrug-resistant human cancer cells contain a specific 150- to 170-kDa protein detected by photoaffinity labeling. Proc Natl Acad Sci USA 83(11):3847–3850
Gottesman MM, Pastan I (1993) Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem 62:385–427. doi:10.1146/annurev.bi.62.070193.002125
Palmeira A, Rodrigues F, Sousa E, Pinto M, Vasconcelos MH, Fernandes MX (2011) New uses for old drugs: pharmacophore-based screening for the discovery of P-glycoprotein inhibitors. Chem Biol Drug Des 78(1):57–72. doi:10.1111/j.1747-0285.2011.01089.x
Chen J, Li Z, Chen AY, Ye X, Luo H, Rankin GO, Chen YC (2013) Inhibitory effect of baicalin and baicalein on ovarian cancer cells. Int J Mol Sci 14(3):6012–6025. doi:10.3390/ijms14036012
Zheng YH, Yin LH, Grahn TH, Ye AF, Zhao YR, Zhang QY (2014) Anticancer effects of baicalein on hepatocellular carcinoma cells. Phytother Res 28(9):1342–1348. doi:10.1002/ptr.5135
Wang Y, Wang Q, Zhang S, Zhang Y, Tao L (2014) Baicalein increases the cytotoxicity of cisplatin by enhancing gap junction intercellular communication. Mol Med Rep 10(1):515–521. doi:10.3892/mmr.2014.2157
Chen F, Zhuang M, Zhong C, Peng J, Wang X, Li J, Chen Z, Huang Y (2015) Baicalein reverses hypoxia-induced 5-FU resistance in gastric cancer AGS cells through suppression of glycolysis and the PTEN/Akt/HIF-1alpha signaling pathway. Oncol Rep 33(1):457–463. doi:10.3892/or.2014.3550
Cho YA, Choi JS, Burm JP (2011) Effects of the antioxidant baicalein on the pharmacokinetics of nimodipine in rats: a possible role of P-glycoprotein and CYP3A4 inhibition by baicalein. Pharmacol Rep 63(4):1066–1073
Sun L, Peng Q, Qu L, Gong L, Si J (2015) Anticancer agent icaritin induces apoptosis through caspase-dependent pathways in human hepatocellular carcinoma cells. Mol Med Rep 11(4):3094–3100. doi:10.3892/mmr.2014.3007
Li S, Priceman SJ, Xin H, Zhang W, Deng J, Liu Y, Huang J, Zhu W, Chen M, Hu W, Deng X, Zhang J, Yu H, He G (2013) Icaritin inhibits JAK/STAT3 signaling and growth of renal cell carcinoma. PLoS One 8(12):e81657. doi:10.1371/journal.pone.0081657
Sun L, Chen W, Qu L, Wu J, Si J (2013) Icaritin reverses multidrug resistance of HepG2/ADR human hepatoma cells via downregulation of MDR1 and Pglycoprotein expression. Mol Med Rep 8(6):1883–1887. doi:10.3892/mmr.2013.1742
Lee KS, Lee HJ, Ahn KS, Kim SH, Nam D, Kim DK, Choi DY, Ahn KS, Lu J, Kim SH (2009) Cyclooxygenase-2/prostaglandin E2 pathway mediates icariside II induced apoptosis in human PC-3 prostate cancer cells. Cancer Lett 280(1):93–100. doi:10.1016/j.canlet.2009.02.024
Sze SC, Tong Y, Ng TB, Cheng CL, Cheung HP (2010) Herba Epimedii: anti-oxidative properties and its medical implications. Molecules 15(11):7861–7870. doi:10.3390/molecules15117861
Liu DF, Li YP, Ou TM, Huang SL, Gu LQ, Huang M, Huang ZS (2009) Synthesis and antimultidrug resistance evaluation of icariin and its derivatives. Bioorg Med Chem Lett 19(15):4237–4240. doi:10.1016/j.bmcl.2009.05.103
Zhang Y, Wang QS, Cui YL, Meng FC, Lin KM (2012) Changes in the intestinal absorption mechanism of icariin in the nanocavities of cyclodextrins. Int J Nanomed 7:4239–4249. doi:10.2147/IJN.S33014
Conseil G, Baubichon-Cortay H, Dayan G, Jault JM, Barron D, Di Pietro A (1998) Flavonoids: a class of modulators with bifunctional interactions at vicinal ATP- and steroid-binding sites on mouse P-glycoprotein. Proc Natl Acad Sci USA 95(17):9831–9836
Zhang S, Morris ME (2003) Effects of the flavonoids biochanin A, morin, phloretin, and silymarin on P-glycoprotein-mediated transport. J Pharmacol Exp Therap 304(3):1258–1267. doi:10.1124/jpet.102.044412
Kim SE, Kim YH, Lee JJ, Kim YC (1998) A new sesquiterpene ester from Celastrus orbiculatus reversing multidrug resistance in cancer cells. J Nat Prod 61(1):108–111. doi:10.1021/np9702392
Munoz-Martinez F, Lu P, Cortes-Selva F, Perez-Victoria JM, Jimenez IA, Ravelo AG, Sharom FJ, Gamarro F, Castanys S (2004) Celastraceae sesquiterpenes as a new class of modulators that bind specifically to human P-glycoprotein and reverse cellular multidrug resistance. Cancer Res 64(19):7130–7138. doi:10.1158/0008-5472.CAN-04-1005
Aggarwal BB, Kumar A, Bharti AC (2003) Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res 23(1A):363–398
Han SS, Chung ST, Robertson DA, Ranjan D, Bondada S (1999) Curcumin causes the growth arrest and apoptosis of B cell lymphoma by downregulation of egr-1, c-myc, bcl-XL, NF-kappa B, and p53. Clin Immunol 93(2):152–161. doi:10.1006/clim.1999.4769
Singh S, Aggarwal BB (1995) Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected]. J Biol Chem 270(42):24995–25000
Anuchapreeda S, Leechanachai P, Smith MM, Ambudkar SV, Limtrakul PN (2002) Modulation of P-glycoprotein expression and function by curcumin in multidrug-resistant human KB cells. Biochem Pharmacol 64(4):573–582
Limtrakul P, Anuchapreeda S, Buddhasukh D (2004) Modulation of human multidrug-resistance MDR-1 gene by natural curcuminoids. BMC Cancer 4:13. doi:10.1186/1471-2407-4-13
Izbicka E, Lawrence R, Raymond E, Eckhardt G, Faircloth G, Jimeno J, Clark G, Von Hoff DD (1998) In vitro antitumor activity of the novel marine agent, ecteinascidin-743 (ET-743, NSC-648766) against human tumors explanted from patients. Ann Oncol 9(9):981–987
Ghielmini M, Colli E, Erba E, Bergamaschi D, Pampallona S, Jimeno J, Faircloth G, Sessa C (1998) In vitro schedule-dependency of myelotoxicity and cytotoxicity of Ecteinascidin 743 (ET-743). Ann Oncol 9(9):989–993
Valoti G, Nicoletti MI, Pellegrino A, Jimeno J, Hendriks H, D’Incalci M, Faircloth G, Giavazzi R (1998) Ecteinascidin-743, a new marine natural product with potent antitumor activity on human ovarian carcinoma xenografts. Clin Cancer Res 4(8):1977–1983
Kanzaki A, Takebayashi Y, Ren XQ, Miyashita H, Mori S, Akiyama S, Pommier Y (2002) Overcoming multidrug drug resistance in P-glycoprotein/MDR1-overexpressing cell lines by ecteinascidin 743. Mol Cancer Ther 1(14):1327–1334
Yang CS, Wang X (2010) Green tea and cancer prevention. Nutr Cancer 62(7):931–937
Lambert JD, Yang CS (2003) Cancer chemopreventive activity and bioavailability of tea and tea polyphenols. Mutat Res 523–524:201–208
Lin Y, Bai L, Chen W, Xu S (2010) The NF-kappaB activation pathways, emerging molecular targets for cancer prevention and therapy. Expert Opin Therap Targets 14(1):45–55. doi:10.1517/14728220903431069
Afaq F, Adhami VM, Ahmad N, Mukhtar H (2003) Inhibition of ultraviolet B-mediated activation of nuclear factor kappaB in normal human epidermal keratinocytes by green tea Constituent (−)-epigallocatechin-3-gallate. Oncogene 22(7):1035–1044. doi:10.1038/sj.onc.1206206
Nomura M, Ma W, Chen N, Bode AM, Dong Z (2000) Inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced NF-kappaB activation by tea polyphenols, (−)-epigallocatechin gallate and theaflavins. Carcinogenesis 21(10):1885–1890
Valente I, Reis M, Duarte N, Serly J, Molnar J, Ferreira MJ (2012) Jatrophane diterpenes from Euphorbia mellifera and their activity as P-glycoprotein modulators on multidrug-resistant mouse lymphoma and human colon adenocarcinoma cells. J Nat Prod 75(11):1915–1921. doi:10.1021/np3004003
Corea G, Di Pietro A, Dumontet C, Fattorusso E, Lanzotti V (2009) Jatrophane diterpenes from Euphorbia spp. as modulators of multidrug resistance in cancer therapy. Phytochem Rev 8(2):431–447. doi:10.1007/s11101-009-9126-8
Shi Z, Jain S, Kim IW, Peng XX, Abraham I, Youssef DT, Fu LW, El Sayed K, Ambudkar SV, Chen ZS (2007) Sipholenol A, a marine-derived sipholane triterpene, potently reverses P-glycoprotein (ABCB1)-mediated multidrug resistance in cancer cells. Cancer Sci 98(9):1373–1380. doi:10.1111/j.1349-7006.2007.00554.x
Abraham I, Jain S, Wu CP, Khanfar MA, Kuang Y, Dai CL, Shi Z, Chen X, Fu L, Ambudkar SV, El Sayed K, Chen ZS (2010) Marine sponge-derived sipholane triterpenoids reverse P-glycoprotein (ABCB1)-mediated multidrug resistance in cancer cells. Biochem Pharmacol 80(10):1497–1506. doi:10.1016/j.bcp.2010.08.001
Kimura S, Ito C, Jyoko N, Segawa H, Kuroda J, Okada M, Adachi S, Nakahata T, Yuasa T, Filho VC, Furukawa H, Maekawa T (2005) Inhibition of leukemic cell growth by a novel anti-cancer drug (GUT-70) from calophyllum brasiliense that acts by induction of apoptosis. Int J Cancer 113(1):158–165. doi:10.1002/ijc.20505
Quesada AR, Garcia Gravalos MD, Fernandez Puentes JL (1996) Polyaromatic alkaloids from marine invertebrates as cytotoxic compounds and inhibitors of multidrug resistance caused by P-glycoprotein. Br J Cancer 74(5):677–682
Zhong Y, Zhang F, Sun Z, Zhou W, Li ZY, You QD, Guo QL, Hu R (2013) Drug resistance associates with activation of Nrf2 in MCF-7/DOX cells, and wogonin reverses it by down-regulating Nrf2-mediated cellular defense response. Mol Carcinog 52(10):824–834. doi:10.1002/mc.21921
Xu X, Zhang Y, Li W, Miao H, Zhang H, Zhou Y, Li Z, You Q, Zhao L, Guo Q (2014) Wogonin reverses multi-drug resistance of human myelogenous leukemia K562/A02 cells via downregulation of MRP1 expression by inhibiting Nrf2/ARE signaling pathway. Biochem Pharmacol 92(2):220–234. doi:10.1016/j.bcp.2014.09.008
Enomoto R, Koshiba C, Suzuki C, Lee E (2011) Wogonin potentiates the antitumor action of etoposide and ameliorates its adverse effects. Cancer Chemother Pharmacol 67(5):1063–1072. doi:10.1007/s00280-010-1396-8
Aoki S, Chen ZS, Higasiyama K, Setiawan A, Akiyama S, Kobayashi M (2001) Reversing effect of agosterol A, a spongean sterol acetate, on multidrug resistance in human carcinoma cells. Jpn J Cancer Res Gann 92(8):886–895
Rabindran SK, Ross DD, Doyle LA, Yang W, Greenberger LM (2000) Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Res 60(1):47–50
Woehlecke H, Osada H, Herrmann A, Lage H (2003) Reversal of breast cancer resistance protein-mediated drug resistance by tryprostatin A. Int J Cancer 107(5):721–728. doi:10.1002/ijc.11444
Huang Q, Huang R, Zhang S, Lin J, Wei L, He M, Zhuo L, Lin X (2013) Protective effect of genistein isolated from Hydrocotyle sibthorpioides on hepatic injury and fibrosis induced by chronic alcohol in rats. Toxicol Lett 217(2):102–110. doi:10.1016/j.toxlet.2012.12.014
Imai Y, Tsukahara S, Asada S, Sugimoto Y (2004) Phytoestrogens/flavonoids reverse breast cancer resistance protein/ABCG2-mediated multidrug resistance. Cancer Res 64(12):4346–4352. doi:10.1158/0008-5472.CAN-04-0078
Wang Y, Zheng J, Liu P, Wang W, Zhu W (2011) Three new compounds from Aspergillus terreus PT06-2 grown in a high salt medium. Mar Drugs 9(8):1368–1378. doi:10.3390/md9081368
Liao WY, Shen CN, Lin LH, Yang YL, Han HY, Chen JW, Kuo SC, Wu SH, Liaw CC (2012) Asperjinone, a nor-neolignan, and terrein, a suppressor of ABCG2-expressing breast cancer cells, from thermophilic Aspergillus terreus. J Nat Prod 75(4):630–635. doi:10.1021/np200866z
Chen YF, Wang SY, Shen H, Yao XF, Zhang FL, Lai D (2014) The marine-derived fungal metabolite, terrein, inhibits cell proliferation and induces cell cycle arrest in human ovarian cancer cells. Int J Mol Med 34(6):1591–1598. doi:10.3892/ijmm.2014.1964
Zeng Y, Zhang Y, Weng Q, Hu M, Zhong G (2010) Cytotoxic and insecticidal activities of derivatives of harmine, a natural insecticidal component isolated from Peganum harmala. Molecules 15(11):7775–7791. doi:10.3390/molecules15117775
Zhang H, Sun K, Ding J, Xu H, Zhu L, Zhang K, Li X, Sun W (2014) Harmine induces apoptosis and inhibits tumor cell proliferation, migration and invasion through down-regulation of cyclooxygenase-2 expression in gastric cancer. Phytomedicine Int J Phytother Phytopharmacol 21(3):348–355. doi:10.1016/j.phymed.2013.09.007
Ma Y, Wink M (2010) The beta-carboline alkaloid harmine inhibits BCRP and can reverse resistance to the anticancer drugs mitoxantrone and camptothecin in breast cancer cells. Phytother Res 24(1):146–149. doi:10.1002/ptr.2860
Huang XC, Xiao X, Zhang YK, Talele TT, Salim AA, Chen ZS, Capon RJ (2014) Lamellarin O, a pyrrole alkaloid from an Australian marine sponge, Ianthella sp., reverses BCRP mediated drug resistance in cancer cells. Mar Drugs 12(7):3818–3837. doi:10.3390/md12073818
Seo J, Lee HS, Ryoo S, Seo JH, Min BS, Lee JH (2011) Tangeretin, a citrus flavonoid, inhibits PGDF-BB-induced proliferation and migration of aortic smooth muscle cells by blocking AKT activation. Eur J Pharmacol 673(1–3):56–64. doi:10.1016/j.ejphar.2011.10.011
Ikegawa T, Ushigome F, Koyabu N, Morimoto S, Shoyama Y, Naito M, Tsuruo T, Ohtani H, Sawada Y (2000) Inhibition of P-glycoprotein by orange juice components, polymethoxyflavones in adriamycin-resistant human myelogenous leukemia (K562/ADM) cells. Cancer Lett 160(1):21–28
Wesolowska O, Wisniewski J, Sroda-Pomianek K, Bielawska-Pohl A, Paprocka M, Dus D, Duarte N, Ferreira MJ, Michalak K (2012) Multidrug resistance reversal and apoptosis induction in human colon cancer cells by some flavonoids present in citrus plants. J Nat Prod 75(11):1896–1902. doi:10.1021/np3003468
Fleisher B, Unum J, Shao J, An G (2015) Ingredients in fruit juices interact with dasatinib through inhibition of BCRP: a new mechanism of beverage-drug interaction. J Pharm Sci 104(1):266–275. doi:10.1002/jps.24289
Steyn PS (1970) The isolation, structure and absolute configuration of secalonic acid D, the toxic metabolite of Penicillium oxalicum. Tetrahedron 26(1):51–57
Hu YP, Tao LY, Wang F, Zhang JY, Liang YJ, Fu LW (2013) Secalonic acid D reduced the percentage of side populations by down-regulating the expression of ABCG2. Biochem Pharmacol 85(11):1619–1625. doi:10.1016/j.bcp.2013.04.003
Tsai P-L, Tsai T-H (2004) Hepatobiliary excretion of berberine. Drug Metab Dispos 32(4):405–412. doi:10.1124/dmd.32.4.405
Letašiová S, Jantová S, Čipák Lu, Múčková M (2006) Berberine—antiproliferative activity in vitro and induction of apoptosis/necrosis of the U937 and B16 cells. Cancer Lett 239(2):254–262. doi:10.1016/j.canlet.2005.08.024
Qi HW, Xin LY, Xu X, Ji XX, Fan LH (2014) Epithelial-to-mesenchymal transition markers to predict response of Berberine in suppressing lung cancer invasion and metastasis. J Transl Med 12(1):22. doi:10.1186/1479-5876-12-22
Park SH, Sung JH, Chung N (2014) Berberine diminishes side population and down-regulates stem cell-associated genes in the pancreatic cancer cell lines PANC-1 and MIA PaCa-2. Mol Cell Biochem 394(1–2):209–215. doi:10.1007/s11010-014-2096-1
Sung JH, Kim JB, Park SH, Park SY, Lee JK, Lee H-S, Chung N (2012) Berberine decreases cell growth but increases the side population fraction of H460 lung cancer cells. J Korean Soc Appl Biol Chem 55(4):491–495. doi:10.1007/s13765-012-2119-0
Li YY, Zhang T (2014) Targeting cancer stem cells by curcumin and clinical applications. Cancer Lett 346(2):197–205. doi:10.1016/j.canlet.2014.01.012
Kakarala M, Brenner DE, Korkaya H, Cheng C, Tazi K, Ginestier C, Liu S, Dontu G, Wicha MS (2010) Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat 122(3):777–785. doi:10.1007/s10549-009-0612-x
Charpentier MS, Whipple RA, Vitolo MI, Boggs AE, Slovic J, Thompson KN, Bhandary L, Martin SS (2014) Curcumin targets breast cancer stem-like cells with microtentacles that persist in mammospheres and promote reattachment. Cancer Res 74(4):1250–1260. doi:10.1158/0008-5472.CAN-13-1778
Mukherjee S, Mazumdar M, Chakraborty S, Manna A, Saha S, Khan P, Bhattacharjee P, Guha D, Adhikary A, Mukhjerjee S, Das T (2014) Curcumin inhibits breast cancer stem cell migration by amplifying the E-cadherin/β-catenin negative feedback loop. Stem Cell Res Therapy 5(5):116. doi:10.1186/scrt506
Chung SS, Vadgama JV (2015) Curcumin and epigallocatechin gallate inhibit the cancer stem cell phenotype via down-regulation of STAT3-NFκB signaling. Anticancer Res 35(1):39
Kanwar SS, Yu Y, Nautiyal J, Patel BB, Padhye S, Sarkar FH, Majumdar APN (2011) Difluorinated-curcumin (CDF): a novel curcumin analog is a potent inhibitor of colon cancer stem-like cells. Pharm Res 28(4):827–838. doi:10.1007/s11095-010-0336-y
Kim EJ, Choi CH, Park JY, Kang SK, Kim YK (2008) Underlying mechanism of quercetin-induced cell death in human glioma cells. Neurochem Res 33(6):971–979. doi:10.1007/s11064-007-9416-8
Duo J, Ying GG, Wang GW, Zhang L (2012) Quercetin inhibits human breast cancer cell proliferation and induces apoptosis via Bcl-2 and Bax regulation. Mol Med Rep 5(6):1453–1456. doi:10.3892/mmr.2012.845
Zhou W, Kallifatidis G, Baumann B, Rausch V, Mattern J, Gladkich J, Giese N, Moldenhauer G, Wirth T, Buchler MW, Salnikov AV, Herr I (2010) Dietary polyphenol quercetin targets pancreatic cancer stem cells. Int J Oncol 37(3):551–561
Chang WW, Hu FW, Yu CC, Wang HH, Feng HP, Lan C, Tsai LL, Chang YC (2013) Quercetin in elimination of tumor initiating stem-like and mesenchymal transformation property in head and neck cancer. Head Neck 35(3):413–419. doi:10.1002/hed.22982
Chen SF, Nieh S, Jao SW, Liu CL, Wu CH, Chang YC, Yang CY, Lin YS (2012) Quercetin suppresses drug-resistant spheres via the p38 MAPK-Hsp27 apoptotic pathway in oral cancer cells. PLoS One 7(11):e49275. doi:10.1371/journal.pone.0049275
Atashpour S, Fouladdel S, Movahhed TK, Barzegar E, Ghahremani MH, Ostad SN, Azizi E (2015) Quercetin induces cell cycle arrest and apoptosis in CD133(+) cancer stem cells of human colorectal HT29 cancer cell line and enhances anticancer effects of doxorubicin. Iran J Basic Med Sci 18(7):635–643
Szkudelski T (2006) Resveratrol inhibits insulin secretion from rat pancreatic islets. Eur J Pharmacol 552(1–3):176–181. doi:10.1016/j.ejphar.2006.09.046
Vanamala J, Charepalli V, Radhakrishnan S, Reddivari L (2012) Resveratrol and grape seed extract combination elevates apoptosis in the colon cancer stem cells, even in the presence of IGF-1, via P53 dependent pathway. FASEB J:26
Shankar S, Nall D, Tang SN, Meeker D, Passarini J, Sharma J, Srivastava RK (2011) Resveratrol inhibits pancreatic cancer stem cell characteristics in human and KrasG12D transgenic mice by inhibiting pluripotency maintaining factors and epithelial-mesenchymal transition. PLoS One 6(1):e16530. doi:10.1371/journal.pone.0016530
Shen YA, Lin CH, Chi WH, Wang CY, Hsieh YT, Wei YH, Chen YJ (2013) Resveratrol impedes the stemness, epithelial-mesenchymal transition, and metabolic reprogramming of cancer stem cells in nasopharyngeal carcinoma through p53 activation. Evid Complement Altern Med 2013:590393. doi:10.1155/2013/590393
Baribeau S, Chaudhry P, Parent S, Asselin E (2014) Resveratrol inhibits cisplatin-induced epithelial-to-mesenchymal transition in ovarian cancer cell lines. PLoS One 9(1):e86987. doi:10.1371/journal.pone.0086987
Fu Y, Chang H, Peng X, Bai Q, Yi L, Zhou Y, Zhu J, Mi M (2014) Resveratrol inhibits breast cancer stem-like cells and induces autophagy via suppressing Wnt/beta-catenin signaling pathway. PLoS One 9(7):e102535. doi:10.1371/journal.pone.0102535
Kocieński PJ, Brown RCD, Pommier A, Procter M, Schmidt B (1998) Synthesis of salinomycin. J Chem Soc Perkin Trans 1(1):9–39. doi:10.1039/a705385a
Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES (2009) Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 138(4):645–659. doi:10.1016/j.cell.2009.06.034
Yue W, Hamai A, Tonelli G, Bauvy C, Nicolas V, Tharinger H, Codogno P, Mehrpour M (2013) Inhibition of the autophagic flux by salinomycin in breast cancer stem-like/progenitor cells interferes with their maintenance. Autophagy 9(5):714–729. doi:10.4161/auto.23997
Chen T, Yi L, Li F, Hu R, Hu S, Yin Y, Lan C, Li Z, Fu C, Cao L, Chen Z, Xian J, Feng H (2015) Salinomycin inhibits the tumor growth of glioma stem cells by selectively suppressing glioma-initiating cells. Mol Med Rep 11(4):2407–2412. doi:10.3892/mmr.2014.3027
Aykut B, Schenk M, Giese N, Kleber S, Martin-Villalba A, Welsch T (2013) Salinomycin is effective against pancreatic cancer stem cells and targets metastasis-promoting fascin. Pancreatology 13(2):e15. doi:10.1016/j.pan.2012.12.105
Mao J, Fan S, Ma W, Fan P, Wang B, Zhang J, Wang H, Tang B, Zhang Q, Yu X, Wang L, Song B, Li L (2014) Roles of Wnt/beta-catenin signaling in the gastric cancer stem cells proliferation and salinomycin treatment. Cell Death Dis 5:e1039. doi:10.1038/cddis.2013.515
Tang QL, Zhao ZQ, Li JC, Liang Y, Yin JQ, Zou CY, Xie XB, Zeng YX, Shen JN, Kang T, Wang J (2011) Salinomycin inhibits osteosarcoma by targeting its tumor stem cells. Cancer Lett 311(1):113–121. doi:10.1016/j.canlet.2011.07.016
Wohlert SE, Künzel E, Machinek R, Méndez C, Salas JA, Rohr J (1999) The structure of mithramycin reinvestigated. J Nat Prod 62(1):119–121. doi:10.1021/np980355k
Zhang M, Mathur A, Zhang Y, Xi S, Atay S, Hong JA, Datrice N, Upham T, Kemp CD, Ripley RT, Wiegand G, Avital I, Fetsch P, Mani H, Zlott D, Robey R, Bates SE, Li X, Rao M, Schrump DS (2012) Mithramycin represses basal and cigarette smoke-induced expression of ABCG2 and inhibits stem cell signaling in lung and esophageal cancer cells. Cancer Res 72(16):4178–4192. doi:10.1158/0008-5472.CAN-11-3983
Leizer AL, Alvero AB, Fu HH, Holmberg JC, Cheng YC, Silasi DA, Rutherford T, Mor G (2011) Regulation of inflammation by the NF-kappa B pathway in ovarian cancer stem cells. Am J Reprod Immunol 65(4):438–447. doi:10.1111/j.1600-0897.2010.00914.x
Don-Doncow N, Escobar Z, Johansson M, Kjellstrom S, Garcia V, Munoz E, Sterner O, Bjartell A, Hellsten R (2014) Galiellalactone Is a direct inhibitor of the transcription factor STAT3 in prostate cancer cells. J Biol Chem 289(23):15969–15978. doi:10.1074/jbc.M114.564252
Hellsten R, Johansson M, Dahlman A, Sterner O, Bjartell A, Pediatrics/Urology/Gynecology/Endocrinology, Sektionen för BUKE, Medicin, Pathology, Department of Laboratory Medicine M, Institutionen för kliniska vetenskaper M, Faculty of M, Department of Clinical Sciences M, Division of Urological C, Urologi, Enheten för urologisk c, Institutionen för laboratoriemedicin M, Lunds u, Lund U, Urology, Patologi M (2011) Galiellalactone inhibits stem cell-like ALDH-positive prostate cancer cells. PloS One 6 (7):e22118. doi:10.1371/journal.pone.0022118
Macha MA, Rachagani S, Gupta S, Pai P, Ponnusamy MP, Batra SK, Jain M (2013) Guggulsterone decreases proliferation and metastatic behavior of pancreatic cancer cells by modulating JAK/STAT and Src/FAK signaling. Cancer Lett 341(2):166–177. doi:10.1016/j.canlet.2013.07.037
Dixit D, Ghildiyal R, Anto NP, Ghosh S, Sharma V, Sen E (2013) Guggulsterone sensitizes glioblastoma cells to Sonic hedgehog inhibitor SANT-1 induced apoptosis in a Ras/NF kappa B dependent manner. Cancer Lett 336(2):347–358. doi:10.1016/j.canlet.2013.03.025
Miyazaki T, Pan Y, Joshi K, Purohit D, Hu B, Demir H, Mazumder S, Okabe S, Yamori T, Viapiano M, Shin-ya K, Seimiya H, Nakano I (2012) Telomestatin impairs glioma stem cell survival and growth through the disruption of telomeric G-quadruplex and inhibition of the proto-oncogene, c-Myb. Clin Cancer Res 18(5):1268–1280. doi:10.1158/1078-0432.CCR-11-1795
Boehmerle W, Muenzfeld H, Springer A, Huehnchen P, Endres M (2014) Specific targeting of neurotoxic side effects and pharmacological profile of the novel cancer stem cell drug salinomycin in mice. J Mol Med 92(8):889–900. doi:10.1007/s00109-014-1155-0
Russo GL, Spagnuolo C, Russo M, Volpe S, Tedesco I, Bilotto S (2012) Synergistic response induced by quercetin and ABT-737 in leukemic cell lines and in B-cells isolated from chronic lymphocytic leukemia. Eur J Cancer 48:S200
Ward AB, Mir H, Kapur N, Singh S (2015) Quercetin inhibits prostate cancer by modulating molecules involved in apoptosis and cell proliferation. Cancer Res. doi:10.1158/1538-7445.AM2015-4642
Borska S, Chmielewska M, Wysocka T, Drag-Zalesinska M, Zabel M, Dziegiel P (2012) In vitro effect of quercetin on human gastric carcinoma: targeting cancer cells death and MDR. Food Chem Toxicol 50(9):3375–3383. doi:10.1016/j.fct.2012.06.035
Srinivasan A, Thangavel C, Liu Y, Shoyele S, Den RB, Selvakumar P, Lakshmikuttyamma A (2015) Quercetin regulates β-catenin signaling and reduces the migration of triple negative breast cancer: qUERCETIN INHIBITS CELL MIGRATION. Mol Carcinog. doi:10.1002/mc.22318
Borska S, Drag-Zalesinska M, Wysocka T, Sopel M, Dumanska M, Zabel M, Dziegiel P (2010) Antiproliferative and pro-apoptotic effects of quercetin on human pancreatic carcinoma cell lines EPP85-181P and EPP85-181RDB. Folia Histochem Cytobiol 48(2):222–229. doi:10.2478/v10042-08-0109-1
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Q., Feng, Y. & Kennedy, D. Multidrug-resistant cancer cells and cancer stem cells hijack cellular systems to circumvent systemic therapies, can natural products reverse this?. Cell. Mol. Life Sci. 74, 777–801 (2017). https://doi.org/10.1007/s00018-016-2362-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-016-2362-3