Abstract
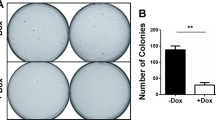
Among the multiple genetic changes that occur during cancer progression are the activation of proto-oncogenes and the inactivation or loss of genes encoding tumor suppressors. The potential roles for these genes in the perturbation of genome stability continues to be of major interest. We have previously shown that conditional expression of H-ras in NIH3T3 cells increases genetic instability in these cells, rendering them more permissive to gene amplification and to the generation of chromosome aberrations which can be induced within a single cell cycle. In the present study we show that genetic instability induced by H-ras expression can be suppressed by co-expressions ofRap 1, aRas-related tumor suppressor gene. An NIH3T3 cell line transformed with activated human H-ras was transfected withRap 1. Expression of theRap 1 gene reverted the transformed cells to a flat morphology. The reverted cells reestablished contact inhibition of growth and lost the capacity to form colonies in soft agar. These cells were subsequently studied for the role ofRap 1 on the suppression of genomic instability induced by oncogenic H-ras. Cells transformed with H-ras manifest an increase in methotrexate resistance as measured by an increase inDhfr gene amplification. Cells which concommitantly expressRap 1 showed reduced levels of methotrexate resistance as well as reduction of gene amplification capacity. Furthermore fluorescent-in-situ hybridization (FISH) with a pancentromeric mouse probe showed that elevated levels of chromosome aberrations in cells expressing H-ras were also suppressed after co-expression ofRap 1.
Similar content being viewed by others
Literature Cited
Foulds, L. (1958). The natural history of cancer.J. Chronic. Dis. 8:2–37.
Fearon, E.R., and Vogelstein, B. (1990). A genetic model for colorectal tumorigenesis.Cell 61:759–767.
Thacker, J. (1985). The molecular nature of mutations in cultured cells: A review.Mutation Res. 150:431–442.
Wolman, S.R. (1983). Karyotypic progression in human tumors.Cancer Metastasis Rev. 2:257–293.
Digweed, M. (1993). Human genetic instability syndromes: Single gene defects with increased risk of cancer.Toxicology Letters 67:259–281.
Bronner, C.E., Baker, S.M., Morrison, P.T., Warren, G., Smith, L.G., Lescoe, M.K., Kane, M., Earabina, C., Lipford, J., Lindblom, A., Tannergard, R., Bolag, R.J., Godwin, A.R., Ward, D.C., Nordenskjold, M., Fishel, R., Kolodner, R., and Liskay, R.M. (1994). Mutations in the DNA mismatch repair gene homolog hMLH1 is associated with hereditary non-polyposis colon cancer.Nature 368:258–261.
Fishel, R., Lescoe, M.K., Rao, M.R.S., Copeland, M.G., Jenkins, N.G., Garber, J., Kane, M., and Kolodner, R. (1993). The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer.Cell 75:1027–1036.
Peltomaki, P., Lothe, R.A., Aaltonen, L.A., Pylkkanen, L., Nystrom-Lathi, M., Seruca, R., David, I., Holm, R., Ryberg, D., Haugen, A., Brogger, A., Borresen, A.L., and de la Chapelle, A. (1993). Microsatellite instability is associated with tumors that characterize the hereditary non-polyposis colorectal carcinoma syndrome.Cancer Res. 53:5853–5855.
Han, H-J., Yanagisawa, A., Kato, Y., Park, J.-G., and Nakamura, Y. (1993). Genetic instability in pancreatic cancer and poorly differentiated type of gastric cancer.Cancer Res. 53:5087–5089.
Mironov, N.M., Aguelon, M.A.-M., Potapova, G.I., Omori, Y., Gorbunov, O.V., Klimenkov, A.A., and Yamasaki, H. (1994). Alterations of (CA)n DNA repeats and tumor supporessor genes in human gastric cancer.Cancer Res. 54:41–44.
Risinger, J.I., Berchuck, A., Kohler, M.F., Watson, P., Lynch, H.T., and Boyd, J. (1993). Genetic instability of microsatellites in endometrial carcinoma.Cancer Res. 53:5100–5103.
Gonzalez-Zulueta, M., Ruppert, J.M., Tokino, K., Tsai, Y.C., Spruck, C.H. III, Miyao, N., Nichols, P.W., Heermann, G.G., Horn, T., Steven, K., Summerhayes, I.C., Sidransky, D., and Jones, P.A. (1993). Microsatellite instability in bladder cancer.Cancer Res. 53:5620–5623.
Livingstone, L.R., White, A., Sprouse, J., Livanose, E., Jacks, T., and Tlsty, T.D. (1992). Altered cell cycle arrest and gene amplification potential accompany loss of wild-type p53.Cell 70:923–935.
Yin, Y., Tainsky, M.A., Bischoff, F.Z., Strong, L.C., and Wahl, G.M. (1992). Wild type p53 restores cell cycle control and inhibits gene amplification in cells with mutant p53 alleles.Cell 70:937–948.
Fukasawa, K., Choi, T., Kuriyama, R., Rulong, S., and Vande Woude, G.F. (1996). Abnormal centrosome amplification in the absence of p53.Science 271:1744–1747.
Wani, M.A., Xu, X., and Stambrook, P.J. (1994). Increased methotrexate resistance and dhfr gene amplification as a consequence of induced Ha-ras expression in NIH 3T3 cells.Cancer Res. 54:2504–2508.
Denko, N.C., Giaccia, A.J., Stringer, J.R., and Stambrook, P.J. (1994). The human Ha-ras oncogene induces genomic instability within one cell cycle.Proc. Natl. Acad. Sci. U.S.A.,91:5124–5128.
Denko, N., Stringer, J., Wani, M., and Stambrook, P.J. (1995). Mitotic and post mitotic consequences of genomic instability induced by oncogenic Ha-Ras.Somatic Cell and Molecular Genetics 21:241–253.
Barbacid, M. (1986). Oncogenes and human cancer: Cause or consequences.Carcinogenesis 7:1037–1042.
Barbacid, M. (1987). ras genes.Annu. Rev. Biochem. 56:779–827.
Forrester, K.C., Almoguera, C., Itan, K., Grïzzle, W.E., and Perucho, M. (1987). Detection of high incidence of K-ras oncogenes during human colon tumorigenesis.Nature 327:298–303.
Corominas, M., Kamino, H., Leon, J., and Pellicer, A. (1989). Oncogene activation in human benign tumors of the skin (keratoacanthomas): isHRAS involved in differention as well as proliferation?Proc. Natl. Acad. Sci. U.S.A. 86:6372–6376.
Ahuja, H.G., Foti, A., Bar-Eli, M., and Cline, M.J. (1990). The pattern of mutational involvement of RAS genes in human hematologic malignancies determined by DNA amplification and direct sequencing.Blood 76:1684–1690.
Land, H., Parada, L.F., and Weinberg, R.A. (1983). Cellular oncogenes and multistep carcinogenesis.Science 222:771–778.
Parada, L.F., Land, H., Weinberg, R.A., Wolf, D., and Rotter, V. (1984). Cooperation between gene encoding p53 tumor antigen and ras in cellular transformation.Nature 312:649–651.
Thompson, T.C., Southgate, J., Kitchener, G., and Land, H. (1989). Multistage carcinogenesis induced by ras and myc oncogenes in a reconstituted organ.Cell 56:917–930.
Tlsty, T.D., White, A., and Sanchez, J. (1992). Suppression of gene amplification in human cell hybrids.Science 255:1425–1427.
Noda, M. (1993). Structures and functions of the Krev-1 transformation suppressor gene and its relatives.Biochem. Biophys. Acta. 1155:97–109.
Sakoda, T., Kaibuchi, K., Kishi, K., Kishida, S., Doi, K., Hoshino, M., Hattori, S., and Takai, Y. (1992).smg/rap 1/krev 1 p21 s inhibit the signal pathway to the c-fos promoter/enhancer from c-ki-ras p21 but not c-raf-1 kinase in NIH3T3 cells.Oncogene 7:1705–1711.
Su, Z., Austin, V.N., Zimmer, S.G., and Fischer, P.B. (1993). Defining the critical gene expression changes associated with expression and suppression of the tumorigenic and metastatic phenotype in Ha-ras transformed cloned rat embryo fibroblasts cells.Oncogene 8:1211–1219.
Sambrook, J., Fritsch, E.F., and Maniatis, T. (eds.). Molecular Cloning: A laboratory manual, New York: Cold Spring Harbor Laboratory press, 1989.
Feinberg, A.P., and Vogelstein, B. (1983). A technique for radiolabelling DNA restriction endonuclease fragment to high specific activity.Anal. Biochem. 132:6–13.
Tlsty, T.D. (1990). Normal diploid human and rodent cells lack a detectable frequency of gene amplification.Proc. Natl. Acad. Sci. U.S.A. 87:3132–3136.
Schimke, R.T. (1984). Gene amplification in cultured animal cells.Cell 37:705–713.
Hamkalo, B.A., Farnham, P.J., Johnston, R., and Schimke, R.T. (1985). Ultrastructural features of minute chromosomes in a methotrexate resistant mouse 3T3 cell line.Proc. Natl. Acad. Sci. U.S.A. 82:1126–1130.
Marx, J. (1993). Forging a path to the nucleus.Science 260:1588–1590.
Feig, L.A. (1993). The many roads that lead to ras.Science 260:767–768.
Wright, J.A., Smith, H.S., Watt, F.M., Hancock, M.C., Hudson, D.L., and Stark, G.R. (1990). DNA amplification is rare in normal human cells.Proc. Natl. Acad. Sci. U.S.A. 87:1791–1795.
Lu, K., and Campisi, J. (1992). RAS proteins are essential and selective for the action of insulin like growth factor 1 late in G1 phase of cell cycle in BALB/c murine fibroblasts.Proc. Natl. Acad. Sci. U.S.A. 89:3889–3893.
Howe, P.H., Dobrowolski, S.F., Reddy, K.B., and Satcey, D.W. (1993). Release from G1 growth arrest by transforming growth factor beta 1 requires cellular ras activity.J. Biol. Chem. 268:21448–21452.
Woessner, R.D., Chung, T.D.Y., Hofmann, G.A., Mattern, M.R., Mirabelli, C.K., Drake, F.H., and Johnson, R.K. (1990). Differences between normal and ras transformed NIH-3T3 cells in expression of the 170 kD and 180 kD forms of topoisomerase II.Cancer Res. 50:2901–2908.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wani, M.A., Denko, N.C. & Stambrook, P.J. Expression ofRap 1 suppresses genomic instability ofH-ras transformed mouse fibroblasts. Somat Cell Mol Genet 23, 123–133 (1997). https://doi.org/10.1007/BF02679971
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02679971